Has a relative mass of 1836 if the mass of an electron is 1. Neutron Proton Electron Has a relative mass of 1839 if the mass of an electron is 1. O Neutron Proton Electron
Has a relative mass of 1836 if the mass of an electron is 1. Neutron Proton Electron Has a relative mass of 1839 if the mass of an electron is 1. O Neutron Proton Electron
Chapter1: Molecular Reasons
Section: Chapter Questions
Problem 4SC
Related questions
Question
Please Help
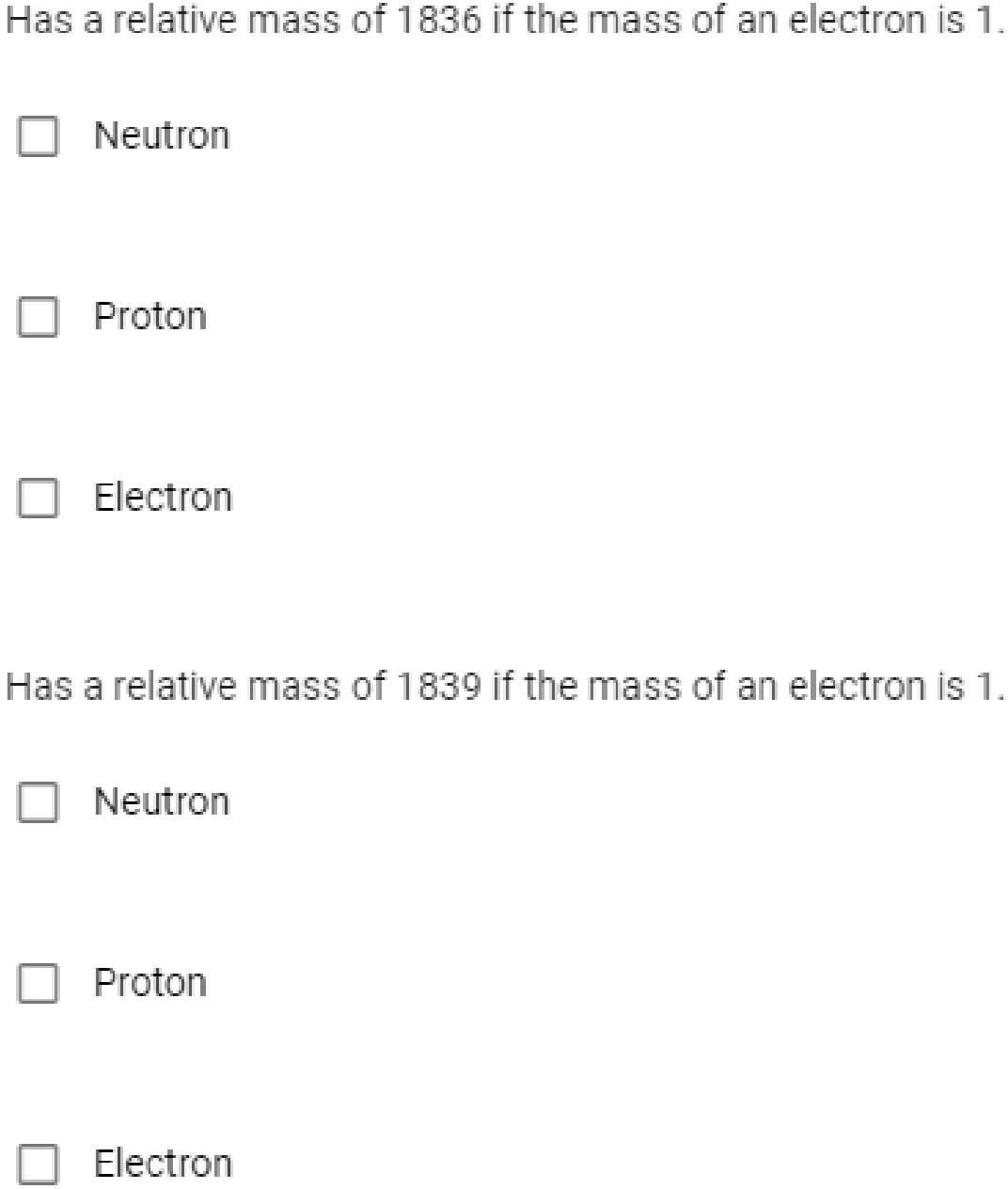
Transcribed Image Text:Has a relative mass of 1836 if the mass of an electron is 1.
Neutron
Proton
Electron
Has a relative mass of 1839 if the mass of an electron is 1.
O Neutron
Proton
Electron
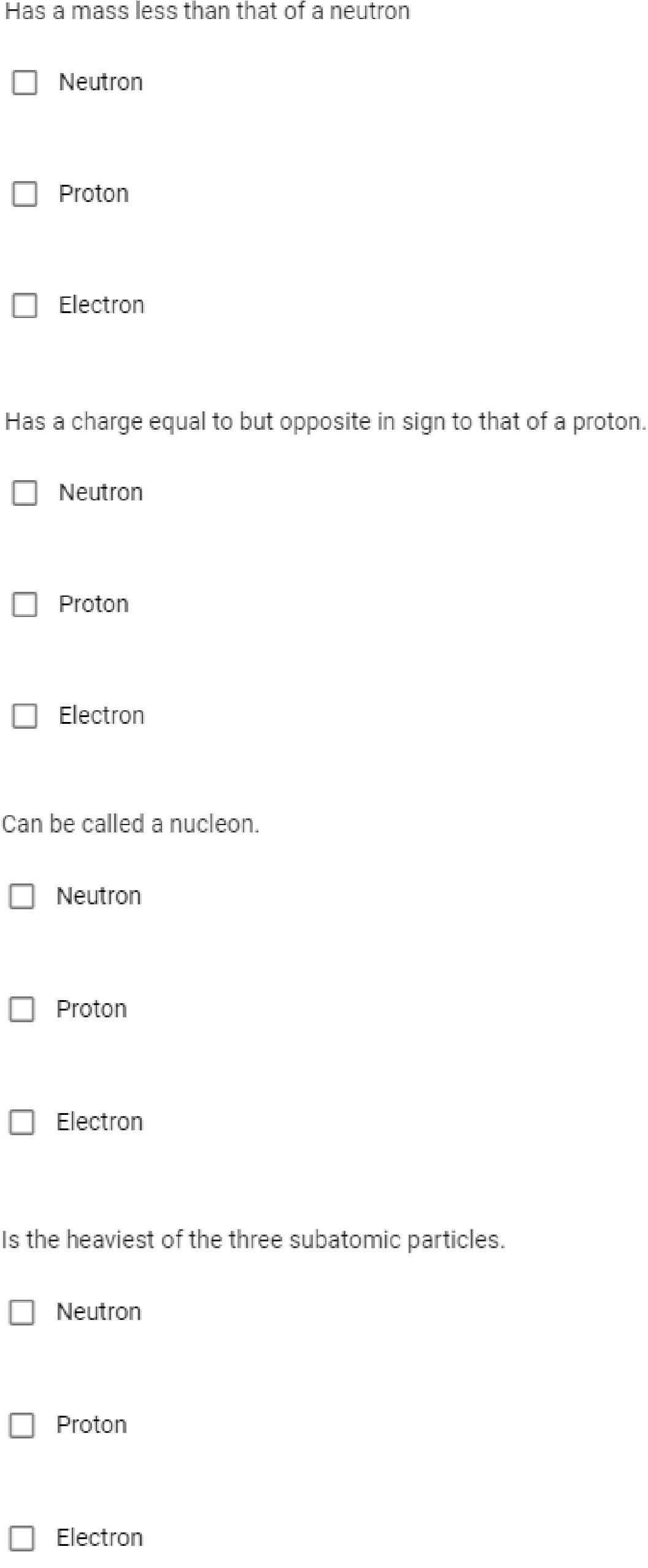
Transcribed Image Text:Has a mass less than that of a neutro
Neutron
Proton
Electron
Has a charge equal to but opposite in sign to that of a proton.
Neutron
Proton
O Electron
Can be called a nucleon.
Neutron
Proton
O Electron
Is the heaviest of the three subatomic particles.
Neutron
Proton
O Electron
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax