HCI(g) can react with methanol vapor, CH;OH(g), to produce CH;CI(g), as represented by the following equation. CH;OH(g) + HCI(g) 2 CH;CI(g) + H2O(g) K, = 4.7x 10° at 400 K CH,OH(g) and HC(g) are combined in a 10.00 L sealed reaction vessel and allowed to reach equilibrium at 400 K. The initial partial pressure of CHOH(g) in the vessel is 0.250 atm and that of HCI(g) is 0.600 atm. (i) Does the total pressure in the vessel increase, decrease, or remain the same as equilibrium is approached? Justify your answer in terms of the reaction stoichiometry.
HCI(g) can react with methanol vapor, CH;OH(g), to produce CH;CI(g), as represented by the following equation. CH;OH(g) + HCI(g) 2 CH;CI(g) + H2O(g) K, = 4.7x 10° at 400 K CH,OH(g) and HC(g) are combined in a 10.00 L sealed reaction vessel and allowed to reach equilibrium at 400 K. The initial partial pressure of CHOH(g) in the vessel is 0.250 atm and that of HCI(g) is 0.600 atm. (i) Does the total pressure in the vessel increase, decrease, or remain the same as equilibrium is approached? Justify your answer in terms of the reaction stoichiometry.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.111P: 5-111 Diving, particularly SCUBA (Self-Contained Underwater Breathing Apparatus) diving, subjects...
Related questions
Question
Could you help explain the first answer in words? I don't know how to explain reaction stoichiometry.

Transcribed Image Text:(ii) Considering the value of K,, calculate the final partial pressure of HCI(g) after the system inside the
vessel reaches equilibrium at 400 K.
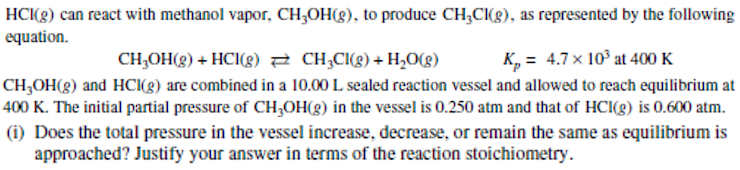
Transcribed Image Text:HC(g) can react with methanol vapor, CH;OH(8), to produce CH;CI(g), as represented by the following
equation.
CH;OH(g) + HCI(g) 2 CH;CI(g) + H2O(g)
K, = 4.7 x 10° at 400 K
CH,OH(g) and HCI(g) are combined in a 10.00 L sealed reaction vessel and allowed to reach equilibrium at
400 K. The initial partial pressure of CH,OH(8) in the vessel is 0.250 atm and that of HCI(g) is 0.600 atm.
(i) Does the total pressure in the vessel increase, decrease, or remain the same as equilibrium is
approached? Justify your answer in terms of the reaction stoichiometry.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning