Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 38P: The energy needed to remove one electron from a gaseous potassium atom is only about two-thirds as...
Related questions
Question
Tell me what do you notice and wonder just write two sentences for each one
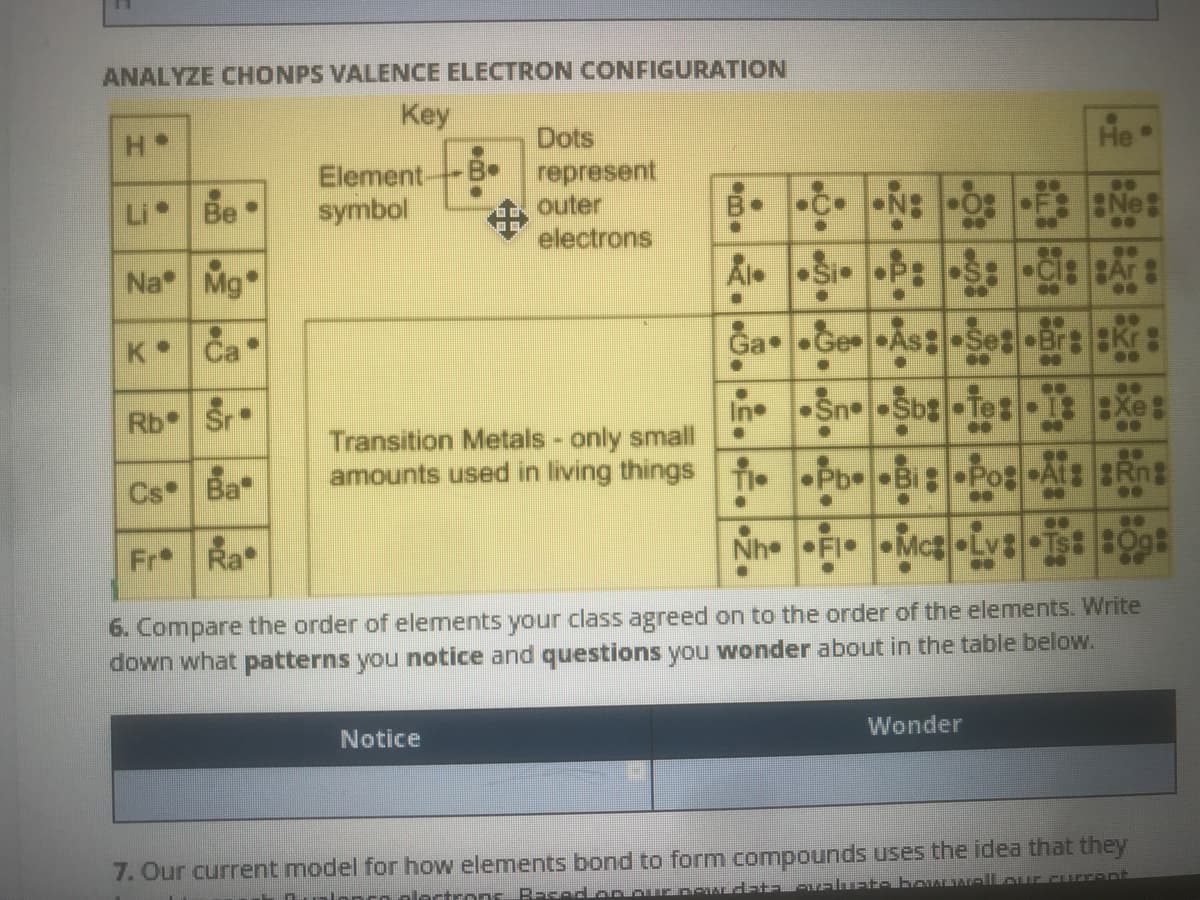
Transcribed Image Text:ANALYZE CHONPS VALENCE ELECTRON CONFIGURATION
Key
Dots
represent
outer
electrons
He•
Element-
symbol
Li Be
Ne:
..
Na Mg
Al. Si P: S:
K Ca
Ga •Ge As: Set •BrEKr
Rb Sr•
•Sn Sb Te:-EXe:
In
Transition Metals - only small
amounts used in living things
Cs Ba
T Pb Bi•Pos At: Rng
TI•
Fr Ra
Nh FI
Mc Lv: Ts: B0g
6. Compare the order of elements your class agreed on to the order of the elements. Write
down what patterns you notice and questions you wonder about in the table below.
Notice
Wonder
7. Our current model for how elements bond to form compounds uses the idea that they
lonco olertrons B-sed on our pow dntaovalTete bowETAalLour current
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning