-heхane-3,4-dlol 5-28 Convert the following perspective formulas to Fischer projections. (а) н он (b) ÇH, (с) Br (d) H OH H он C-C CH, CH3 CH,OH Br H СНО CH, НОСН, HOCH, н он
-heхane-3,4-dlol 5-28 Convert the following perspective formulas to Fischer projections. (а) н он (b) ÇH, (с) Br (d) H OH H он C-C CH, CH3 CH,OH Br H СНО CH, НОСН, HOCH, н он
Chapter7: Alkenes: Structure And Reactivity
Section7.SE: Something Extra
Problem 24VC: The following carbocation is an intermediate in the electrophilic addition reaction of HCl with two...
Related questions
Question
100%
The ff. questions are atttached:
a.
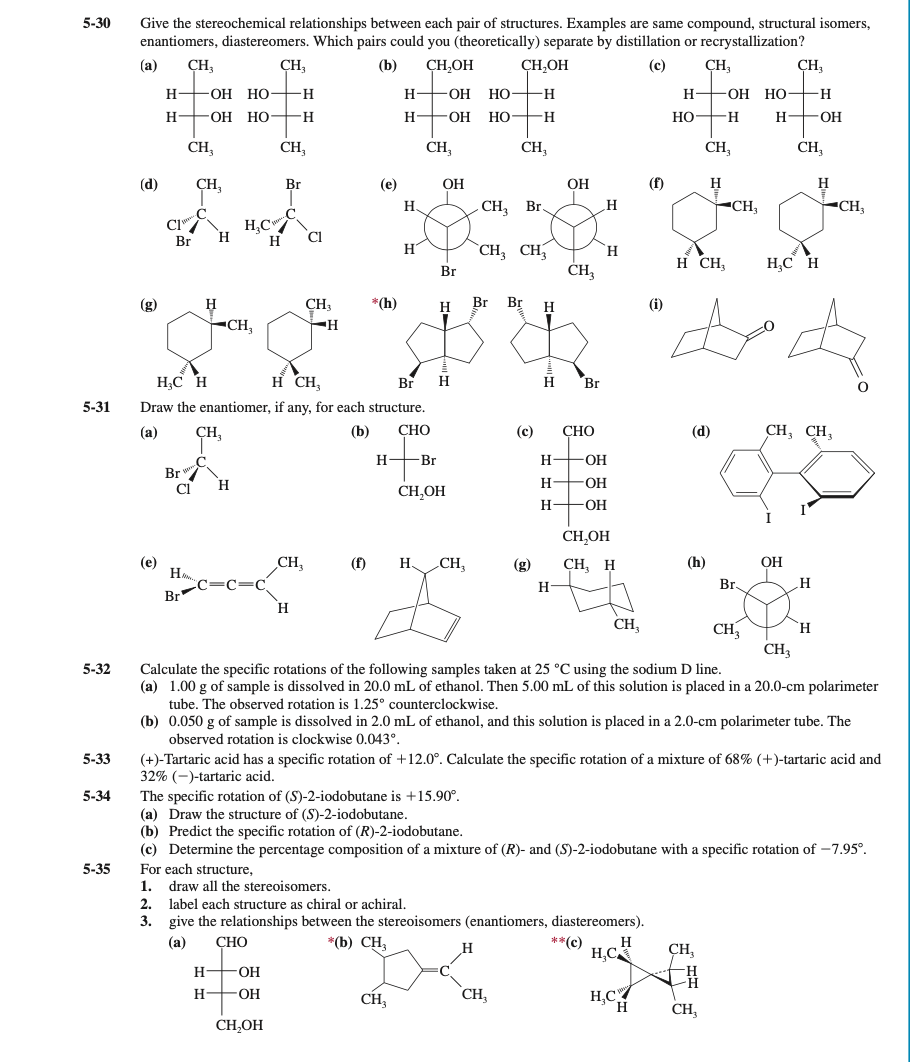
Transcribed Image Text:5-30
Give the stereochemical relationships between each pair of structures. Examples are same compound, structural isomers,
enantiomers, diastereomers. Which pairs could you (theoretically) separate by distillation or recrystallization?
(a)
CH3
CH,
(b)
CH,OH
CH,OH
(c)
CH,
CH,
H-
—ОН НО-
-H
H-
-O-
Но-
-H-
Н—он НО—н
H
ОН НО
H.
H
-OH-
HO-
-H-
Но —н
H FOH
CH,
CH,
CH,
CH,
CH,
CH,
(d)
Br
(e)
OH
ОН
(f)
H
H
Н.
CH, Br.
CH,
-CH,
C.
CI
.C
H,C
H
H
Br
H
CH, CH,
ĊH
H.
H CH,
H,C H
Br
(g)
H
CH3
*(h)
H
Br
Br
H
(i)
-CH,
H;C H
H CH,
Br
H
Br
5-31
Draw the enantiomer, if any, for each structure.
(а)
CH,
(b)
СНО
(c)
СНО
(d)
CH, CH,
H
-Br
H
OH
Br
H
H-
-OH
CI
CH,OH
H
-O-
CH,OH
(e)
H
CH,
(f)
H CH,
(g)
CH, H
(h)
ОН
H-
Br,
H
Br
H
CH,
CH,
`H
CH3
5-32
Calculate the specific rotations of the following samples taken at 25 °C using the sodium D line.
(a) 1.00 g of sample is dissolved in 20.0 mL of ethanol. Then 5.00 mL of this solution is placed in a 20.0-cm polarimeter
tube. The observed rotation is 1.25° counterclockwise.
(b) 0.050 g of sample is dissolved in 2.0 mL of ethanol, and this solution is placed in a 2.0-cm polarimeter tube. The
observed rotation is clockwise 0.043°.
(+)-Tartaric acid has a specific rotation of +12.0°. Calculate the specific rotation of a mixture of 68% (+)-tartaric acid and
32% (-)-tartaric acid.
The specific rotation of (S)-2-iodobutane is +15.90°.
(a) Draw the structure of (S)-2-iodobutane.
(b) Predict the specific rotation of (R)-2-iodobutane.
(c) Determine the percentage composition of a mixture of (R)- and (S)-2-iodobutane with a specific rotation of –7.95°.
5-33
5-34
5-35
For each structure,
1.
draw all the stereoisomers.
2. label each structure as chiral or achiral.
3. give the relationships between the stereoisomers (enantiomers, diastereomers).
(а)
СНО
*(b) CH,
**(c)
H
H,C
CH,
H-
-OH
CH,
H,C
H
H-
-OH-
CH,
CH,
CH,OH
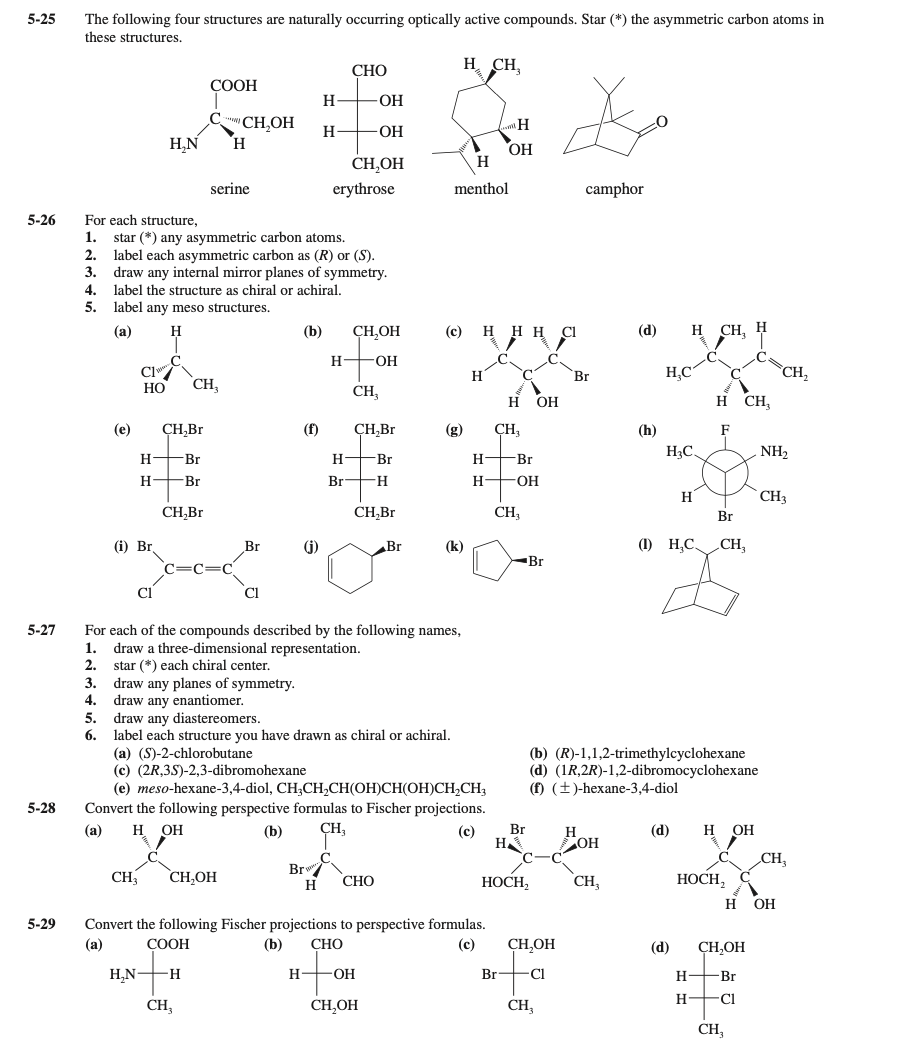
Transcribed Image Text:5-25
The following four structures are naturally occurring optically active compounds. Star (*) the asymmetric carbon atoms in
these structures.
СНО
H CH,
СООН
H
ОН
C CH,OH
H,N
H
H
-ОН
H.
OH
CH,OH
serine
erythrose
menthol
camphor
5-26
For each structure,
1.
star (*) any asymmetric carbon atoms.
2. label each asymmetric carbon as (R) or (S).
3. draw any internal mirror planes of symmetry.
4. label the structure as chiral or achiral.
5.
label any meso structures.
(а)
H
(b)
ÇH,OH
(с)
H.
(d)
Н СH, Н
H
FHO-
Cl
CH,
НО
H,C
Br
CH,
CH,
н он
H CH,
(e)
ÇH,Br
(f)
ÇH,Br
(g)
ÇH,
(h)
H3C.
NH2
H Br
Н— Br
Н— Br
H +Br
Br —н
H FOH
H
CH3
CH,Br
CH,Br
CH,
Br
(i) Br.
Br
(j)
Br
(k)
(1) H,C.
CH,
Br
Cl
For each of the compounds described by the following names,
draw a three-dimensional representation.
2.
5-27
1.
star (*) each chiral center.
3. draw any planes of symmetry.
4. draw any enantiomer.
5. draw any diastereomers.
6.
label each structure you have drawn as chiral or achiral.
(a) (S)-2-chlorobutane
(c) (2R,3S)-2,3-dibromohexane
(е) тeso-hexane-3,4-diol, CH,CH,CН(ОНІСН(ОН)СН,CH,
(b) (R)-1,1,2-trimethylcyclohexane
(d) (1R,2R)-1,2-dibromocyclohexane
(f) (±)-hexane-3,4-diol
5-28
Convert the following perspective formulas to Fischer projections.
H
(а)
OH
(b)
ÇH;
(c)
Br
H
(d)
н Он
OH
CH,
Br
CH
CH,OH
НОСН,
CH,
НОСН, С
H
СНО
н он
5-29
Convert the following Fischer projections to perspective formulas.
(a)
СООН
(b)
СНО
(c)
CH,OH
(d)
CH,OH
H,N-H
H-
-O-
Br
FC1
Н— Br
H +CI
CH,
CH,OH
CH,
CH,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning