hello i am having difficulty with part a, b, and c A galvanic cell based on the above reaction is constructed according to the generic sketch of a galvanic cell shown in the picture attached below. 1a) Choose the letter from the picture which best describes the following: Solution containing IO3−, I2 and source of H+ Pt cathode 1b) which of the following overall, balanced, spontaneous reaction is occurring in this (standard) cell? 12H+(aq) + 2IO3−(aq) + 10Fe2+(aq) → 10Fe3+(aq) + I2(s) + 6H2O(l) 2IO3−(aq) + 10Fe2+(aq) → 10Fe3+(aq) + I2(s) + 6H2O(l) 12H+(aq) + 2IO3−(aq) + 2Fe2+(aq) → 2Fe3+(aq) + I2(s) + 6H2O(l) 10Fe3+(aq) + I2(s) + 6H2O(l) → 12H+(aq) + 2IO3−(aq) + 10Fe2+(aq) 1c) true or false? The salt bridge is intended to prevent counter ions from flowing between the two cell compartments. The salt bridge is labeled 'D'. Tube 'D' hinders Fe2+ ions from migrating to the other compartment and reacting directly with the iodate solution. The salt bridge completes the electrical circuit while separating the anode and cathode compartments. The solution in tube 'D' must contain an electrolyte for the cell to operate.
hello i am having difficulty with part a, b, and c
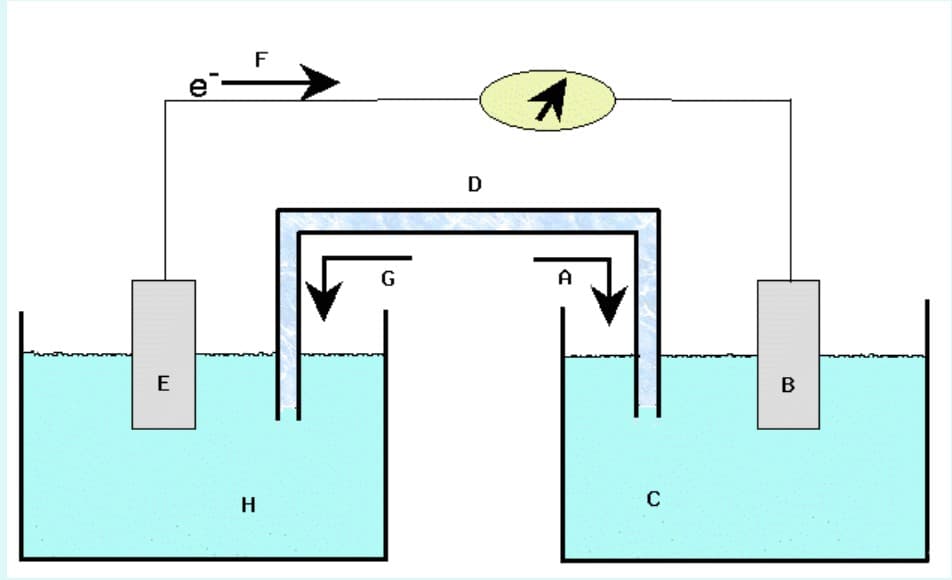
A galvanic cell based on the above reaction is constructed according to the generic sketch of a galvanic cell shown in the picture attached below.
1a) Choose the letter from the picture which best describes the following:
Solution containing IO3−, I2 and source of H+
Pt cathode
1b) which of the following overall, balanced, spontaneous reaction is occurring in this (standard) cell?
12H+(aq) + 2IO3−(aq) + 10Fe2+(aq) → 10Fe3+(aq) + I2(s) + 6H2O(l)
2IO3−(aq) + 10Fe2+(aq) → 10Fe3+(aq) + I2(s) + 6H2O(l)
12H+(aq) + 2IO3−(aq) + 2Fe2+(aq) → 2Fe3+(aq) + I2(s) + 6H2O(l)
10Fe3+(aq) + I2(s) + 6H2O(l) → 12H+(aq) + 2IO3−(aq) + 10Fe2+(aq)
1c) true or false?
The salt bridge is intended to prevent counter ions from flowing between the two cell compartments.
The salt bridge is labeled 'D'.
Tube 'D' hinders Fe2+ ions from migrating to the other compartment and reacting directly with the iodate solution.
The salt bridge completes the electrical circuit while separating the anode and cathode compartments.
The solution in tube 'D' must contain an electrolyte for the cell to operate.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps


