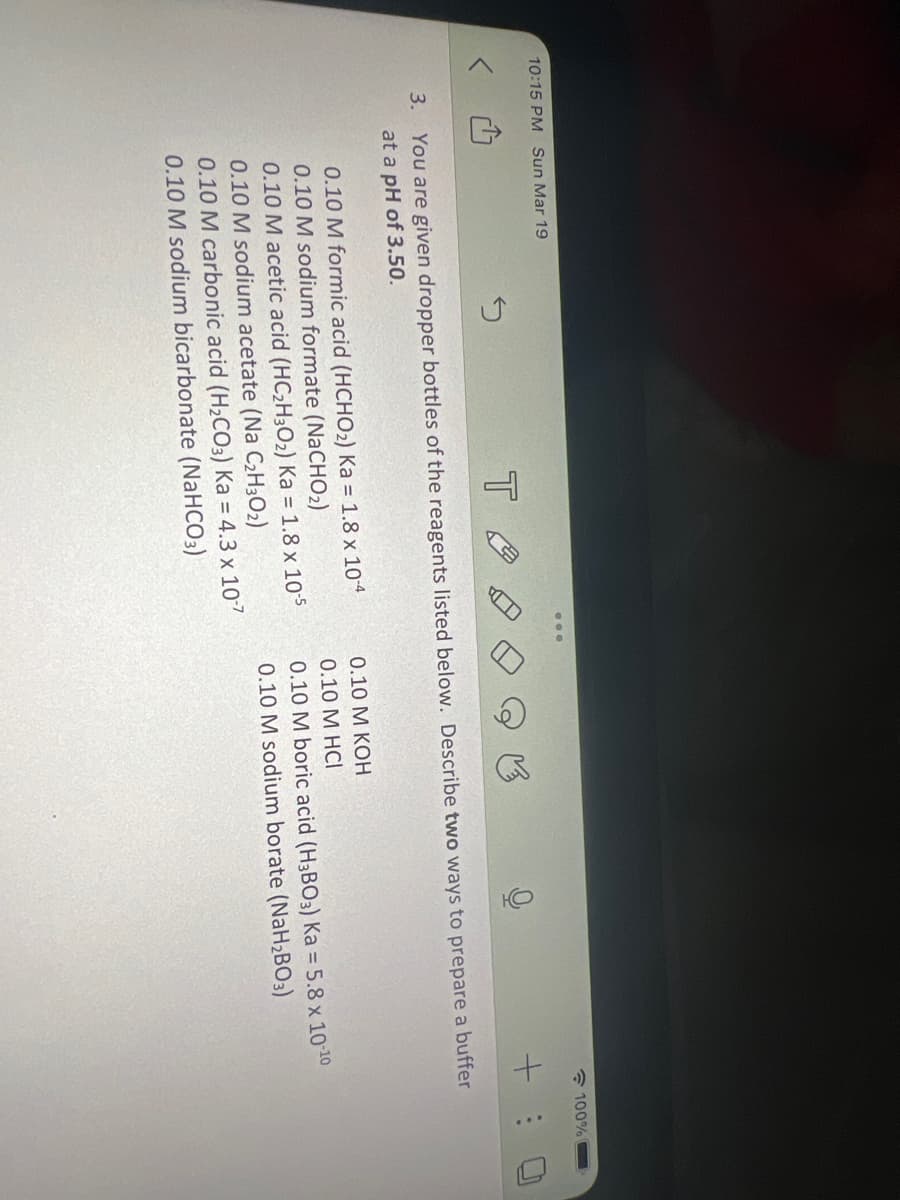
5 You are given dropper bottles of the reagents listed below. Describe two ways to prepare a buffer at a pH of 3.50. 0.10 M formic acid (HCHO₂) Ka = 1.8 x 10-4 0.10 M sodium formate (NaCHO₂) 0.10 M acetic acid (HC₂H30₂) Ka = 1.8 x 10-5 0.10 M sodium acetate (Na C₂H30₂) 0.10 M carbonic acid (H₂CO3) Ka = 4.3 x 10-7 0.10 M sodium bicarbonate (NaHCO3) 0.10 M KOH 0.10 M HCI 0.10 M boric acid (H3BO3) Ka = 5.8 x 10-10 0.10 M sodium borate (NaH₂BO3)
5 You are given dropper bottles of the reagents listed below. Describe two ways to prepare a buffer at a pH of 3.50. 0.10 M formic acid (HCHO₂) Ka = 1.8 x 10-4 0.10 M sodium formate (NaCHO₂) 0.10 M acetic acid (HC₂H30₂) Ka = 1.8 x 10-5 0.10 M sodium acetate (Na C₂H30₂) 0.10 M carbonic acid (H₂CO3) Ka = 4.3 x 10-7 0.10 M sodium bicarbonate (NaHCO3) 0.10 M KOH 0.10 M HCI 0.10 M boric acid (H3BO3) Ka = 5.8 x 10-10 0.10 M sodium borate (NaH₂BO3)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter15: Additional Aqueous Equilibria
Section: Chapter Questions
Problem 15.BCP
Related questions
Question
Transcribed Image Text:10:15 PM Sun Mar 19
3.
0.10 M formic acid (HCHO₂) Ka = 1.8 x 10-4
0.10 M sodium formate (NaCHO₂)
5
TA
You are given dropper bottles of the reagents listed below. Describe two ways to prepare a buffer
at a pH of 3.50.
0.10 M acetic acid (HC₂H30₂) Ka = 1.8 x 10-5
0.10 M sodium acetate (Na C₂H30₂)
0.10 M carbonic acid (H₂CO3) Ka = 4.3 x 10-7
0.10 M sodium bicarbonate (NaHCO3)
100%
+ :
0.10 M KOH
0.10 M HCI
0.10 M boric acid (H3BO3) Ka = 5.8 x 10-10
0.10 M sodium borate (NaH₂BO3)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning