Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 1CR
Related questions
Question
Help
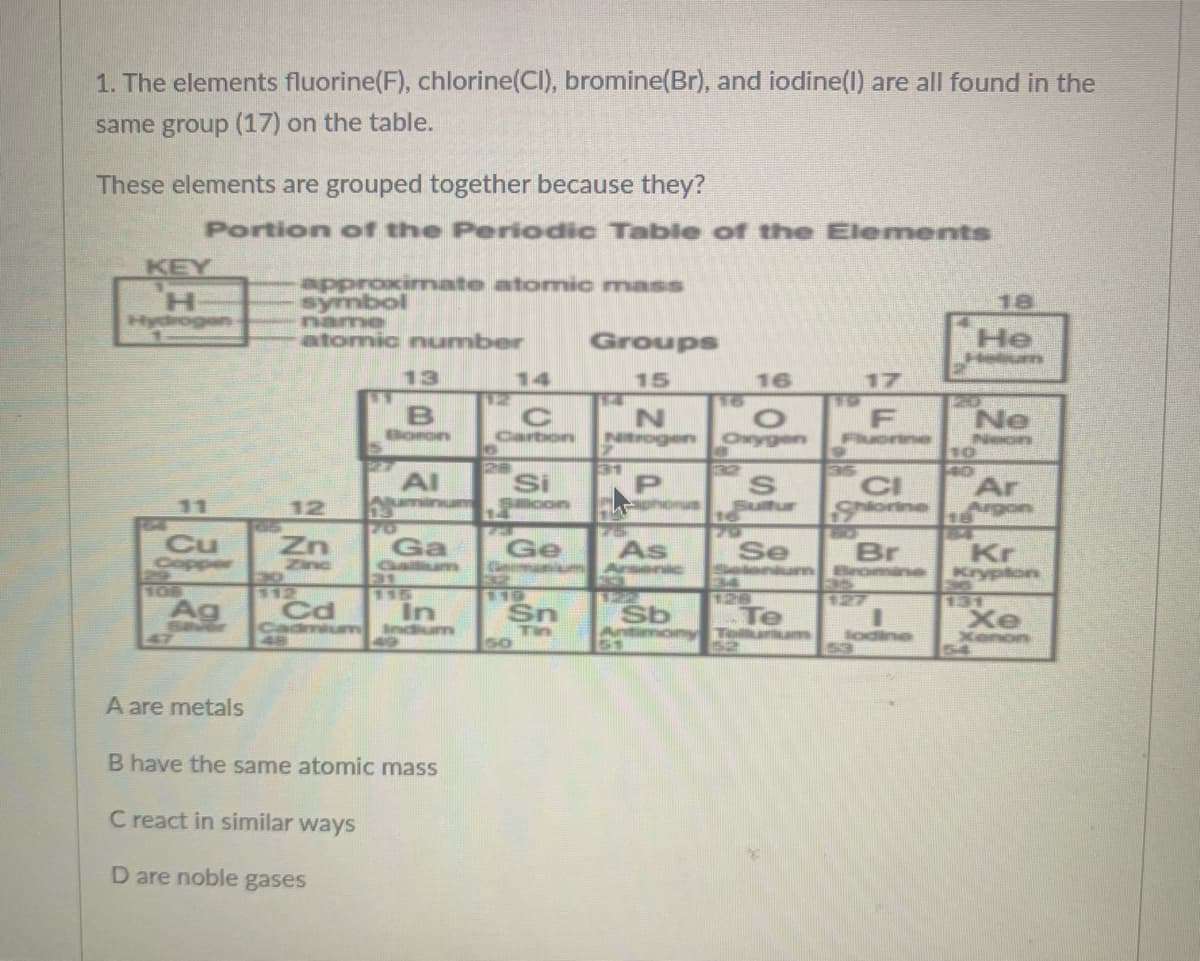
Transcribed Image Text:1. The elements fluorine(F), chlorine(Cl), bromine(Br), and iodine(l) are all found in the
same group (17) on the table.
These elements are grouped together because they?
Portion of the Periodic Table of the Elements
KEY
H.
Hydrogen
approxirmate atomic mass
symbol
name
atomic number
18
Groups
He
Helum
13
14
15
16
17
Ne
Neon
Boron
Carbon
Ntrogen
Oxygen
Al
Auminum
Si
con
CI
Slorine
Ar
Argon
11
12
Suitur
720
Ga
Gallum
Cu
Ge
Cmnm Aenic
As
Se
Zinc
EXD
Br
Seleniun Bromine
35
Kr
Krypton
106
116
In
andium
131
Xe
Xenon
54
Sav
47
Ag
Cd
Cadmium
Sn
Te
Telurum
Tin
so
Antimony
51
lodine
53
A are metals
B have the same atomic mass
C react in similar ways
are noble gases
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax