Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter8: Bonding And Molecular Structure
Section: Chapter Questions
Problem 92IL: Uracil is one of the bases in RNA, a close relative of DNA. (a) What are the values of the OCN and...
Related questions
Question
Help filling in Lewis structure graph
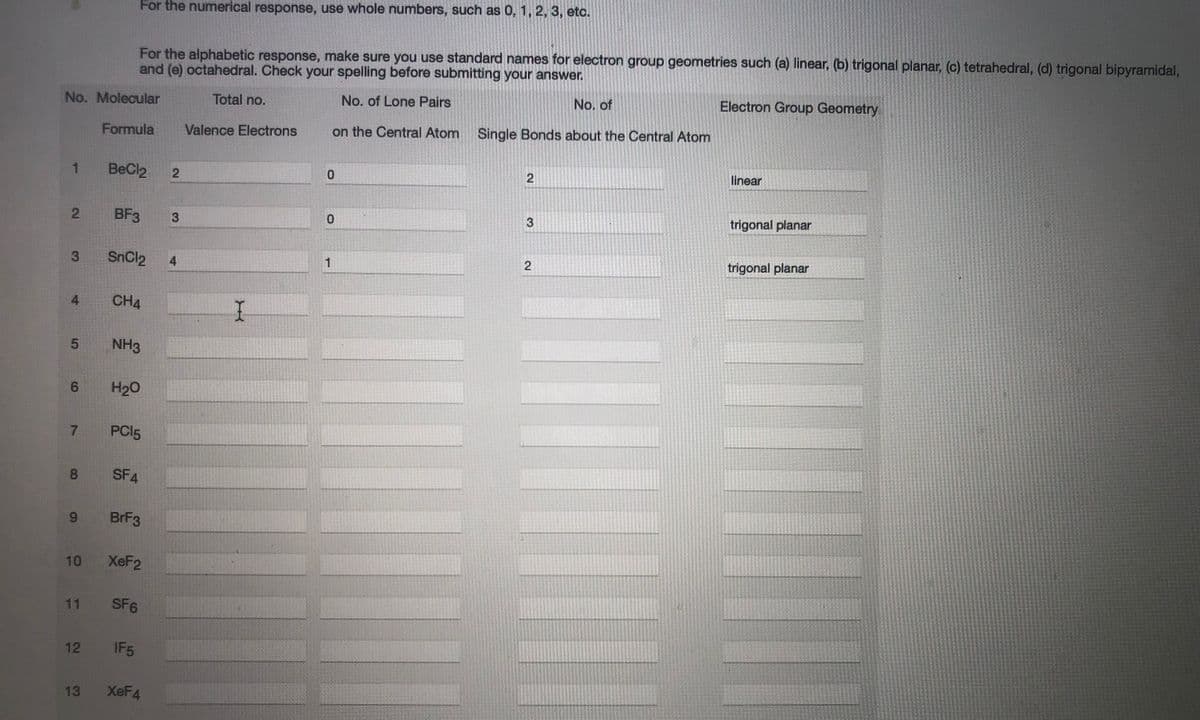
Transcribed Image Text:For the numerical response, use whole numbers, such as 0, 1, 2, 3, etc.
For the alphabetic response, make sure you use standard names for electron group geometries such (a) linear, (b) trigonal planar, (c) tetrahedral, (d) trigonal bipyramidal,
and (e) octahedral. Check your spelling before submitting your answer.
No. Molecular
Total no.
No. of Lone Pairs
No. of
Electron Group Geometry
Formula
Valence Electrons
on the Central Atom
Single Bonds about the Central Atom
1
BeCl2
linear
BF3
trigonal planar
3
SnCl2
trigonal planar
4
1
4.
CH4
NH3
6.
H20
7.
PCI5
8.
SF4
BRF3
10 XEF2
11 SF6
12 IF5
13
XEF4
2.
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning