Ammonia can also be synthesized by the reaction: 3H,(g) + N2(g) →2NH;(8) What is the theoretical yield of ammonia, in kilograms, that we can synthesize from 5.22 kg of H2 and 33.6 kg of N2? ΑΣΦ theoretical yield of NH3 = kg
Ammonia can also be synthesized by the reaction: 3H,(g) + N2(g) →2NH;(8) What is the theoretical yield of ammonia, in kilograms, that we can synthesize from 5.22 kg of H2 and 33.6 kg of N2? ΑΣΦ theoretical yield of NH3 = kg
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter21: The Chemistry Of The Main Group Elements
Section21.11: The Noble Gases, Group 8a
Problem 2.4ACP: The best catalysts used to accelerate the decomposition of ammonia borane result in release of 140 g...
Related questions
Question
Ammonia can also be synthesized by the reaction:
3H2(g)+N2(g)→2NH3(g)3H2(g)+N2(g)→2NH3(g)
What is the theoretical yield of ammonia, in kilograms, that we can synthesize from 5.22 kgkg of H2H2 and 33.6 kgkg of N2N2?
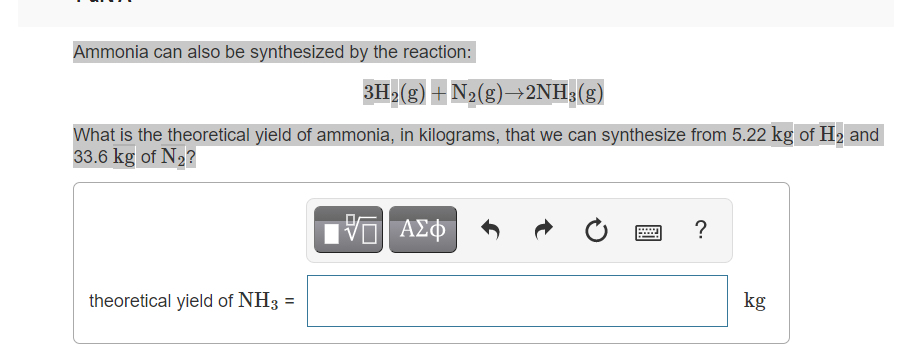
Transcribed Image Text:Ammonia can also be synthesized by the reaction:
3H>(g) + N2(g)→2NH;(g)
What is the theoretical yield of ammonia, in kilograms, that we can synthesize from 5.22 kg of H2 and
33.6 kg of N2?
η ΑΣΦ
theoretical yield of NH3
kg
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning