hematite in Sedona, AZ Fe₂0₂ stacked magnetite rocks Fe₂O Two of the most common forms of tron oxides found in the Earth's crust are hematite, Fe,O, and magnetite, Fe,O.. Although Fe and O are not commonly encountered in the semiconductors we have talked about so far, both are actually semiconductors, and also minerals (rocks!). Hematite is red, and s be observed in spectacular form in Sedona, Arizona, which is about 120 miles north of Arizona State University. rid, is almost black. A substance will appear red to a viewer if when light hits it, the higher energy blue light is the lower c ergy red light is not absorbed, but is reflected off towards the viewer. A substance will appear black to a viewer if when light hits it all of the light is absorbed by the substance, including the lower energy red light, and none is reflected off towards the viewer Magnetite absorbed by t Given the list of options, select the correct answer to this question: Which has the larger band gap, hematite or magnetite? Hematite has the larger band gap and magnetite the smaller band gap. Magnetite has the larger band gap and hematite the smaller band gap Save
hematite in Sedona, AZ Fe₂0₂ stacked magnetite rocks Fe₂O Two of the most common forms of tron oxides found in the Earth's crust are hematite, Fe,O, and magnetite, Fe,O.. Although Fe and O are not commonly encountered in the semiconductors we have talked about so far, both are actually semiconductors, and also minerals (rocks!). Hematite is red, and s be observed in spectacular form in Sedona, Arizona, which is about 120 miles north of Arizona State University. rid, is almost black. A substance will appear red to a viewer if when light hits it, the higher energy blue light is the lower c ergy red light is not absorbed, but is reflected off towards the viewer. A substance will appear black to a viewer if when light hits it all of the light is absorbed by the substance, including the lower energy red light, and none is reflected off towards the viewer Magnetite absorbed by t Given the list of options, select the correct answer to this question: Which has the larger band gap, hematite or magnetite? Hematite has the larger band gap and magnetite the smaller band gap. Magnetite has the larger band gap and hematite the smaller band gap Save
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.96PAE: 8.96 A business manager wants to provide a wider range of p- and n-type semiconductors as a strategy...
Related questions
Question
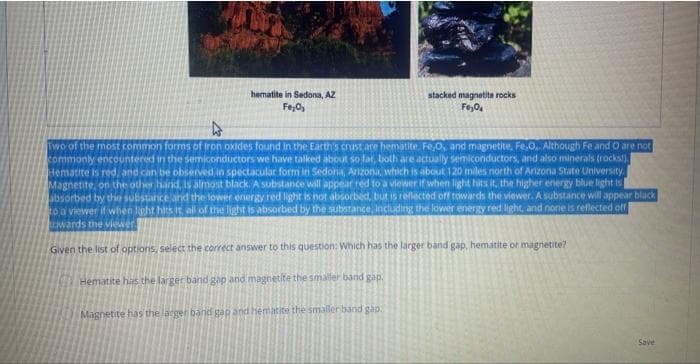
Transcribed Image Text:hematite in Sedona, AZ
Fe₂O₂
stacked magnetite rocks
Fe₂04
Two of the most common forms of iron oxides found in the Earth's crust are hematite, Fe,O, and magnetite, Fe,O.. Although Fe and O are not
commonly encountered in the semiconductors we have talked about so far, both are actually semiconductors, and also minerals (rocks!).
Hematite is red, and can be observed in spectacular form in Sedona, Arizona, which is about 120 miles north of Arizona State University.
Magnetite, on the other hand, is almost black. A substance will appear red to a viewer if when light hits it, the higher energy blue light is
absorbed by the substance and the lower energy red light is not absorbed, but is reflected off towards the viewer. A substance will appear black
to a viewer if when light hits it all of the light is absorbed by the substance, including the lower energy red light, and none is reflected off
towards the viewer.
Given the list of options, select the correct answer to this question: Which has the larger band gap, hematite or magnetite?
Hematite has the larger band gap and magnetite the smaller band gap.
Magnetite has the larger band gap and hematite the smaller band gap
Save
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax