Here is a graph of the probability of an atom moving with a particular speed, for a sample of neon gas at – 144. °C. probability 100 200 300 400 500 600 700 800 900 speed (m/s) Use this graph to answer the following questions. Round each of your answers to the nearest m/s. Note: your answers must be within 25 m/s of the exact answers to be graded correct. What is the most likely speed of a Ne atom in this sample? What higher speed is only half as likely as the most likely speed? What higher speed is only 10% as likely as the most likely speed?
Here is a graph of the probability of an atom moving with a particular speed, for a sample of neon gas at – 144. °C. probability 100 200 300 400 500 600 700 800 900 speed (m/s) Use this graph to answer the following questions. Round each of your answers to the nearest m/s. Note: your answers must be within 25 m/s of the exact answers to be graded correct. What is the most likely speed of a Ne atom in this sample? What higher speed is only half as likely as the most likely speed? What higher speed is only 10% as likely as the most likely speed?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section: Chapter Questions
Problem 122QRT
Related questions
Question
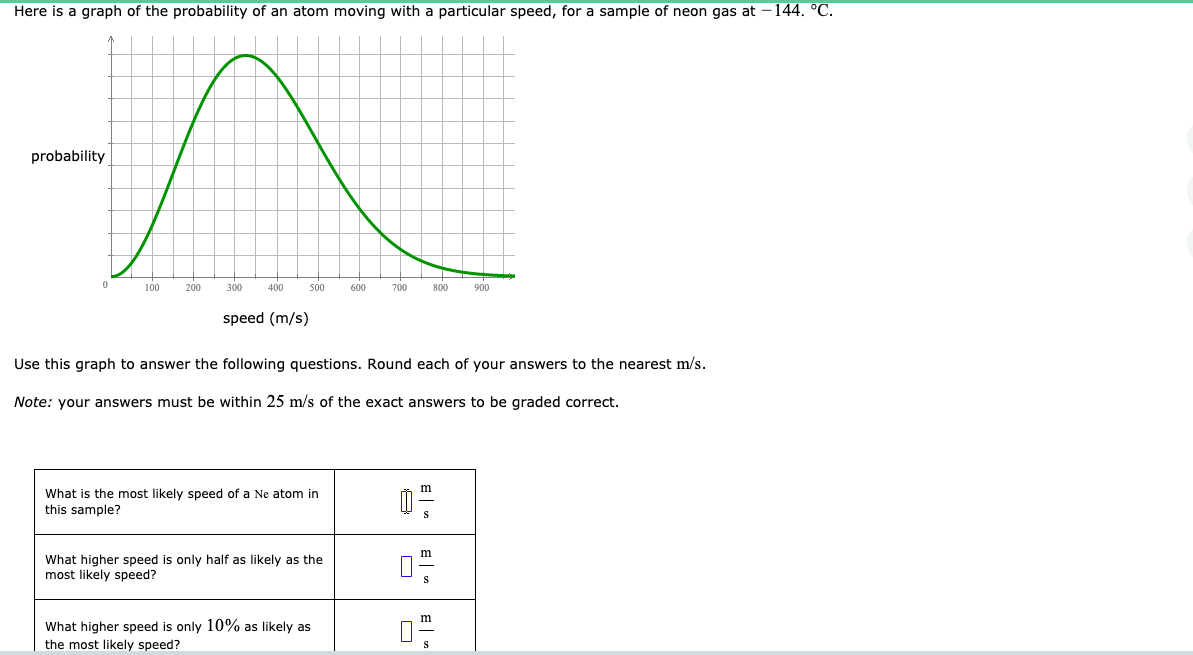
Transcribed Image Text:Here is a graph of the probability of an atom moving with a particular speed, for a sample of neon gas at – 144. °C.
probability
100
200
300
400
500
600
700
800
900
speed (m/s)
Use this graph to answer the following questions. Round each of your answers to the nearest m/s.
Note: your answers must be within 25 m/s of the exact answers to be graded correct.
What is the most likely speed of a Ne atom in
this sample?
What higher speed is only half as likely as the
most likely speed?
What higher speed is only 10% as likely as
the most likely speed?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning