Here is oh of the probability of an atom moving with a particular speed, for a sample of neon gas at –157. °C. probability 100 200 300 400 500 600 700 800 900 speed (m/s) Use this graph to answer the following questions. Round each of your answers to the nearest m/s. Note: your answers must be within 25 m/s of the exact answers to be graded correct. m What is the most likely speed of a Ne atom in this sample? What higher speed is only half as likely as the most likely speed? S m What higher speed is only 10% as likely as the most likely speed? S O
Here is oh of the probability of an atom moving with a particular speed, for a sample of neon gas at –157. °C. probability 100 200 300 400 500 600 700 800 900 speed (m/s) Use this graph to answer the following questions. Round each of your answers to the nearest m/s. Note: your answers must be within 25 m/s of the exact answers to be graded correct. m What is the most likely speed of a Ne atom in this sample? What higher speed is only half as likely as the most likely speed? S m What higher speed is only 10% as likely as the most likely speed? S O
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section: Chapter Questions
Problem 122QRT
Related questions
Question
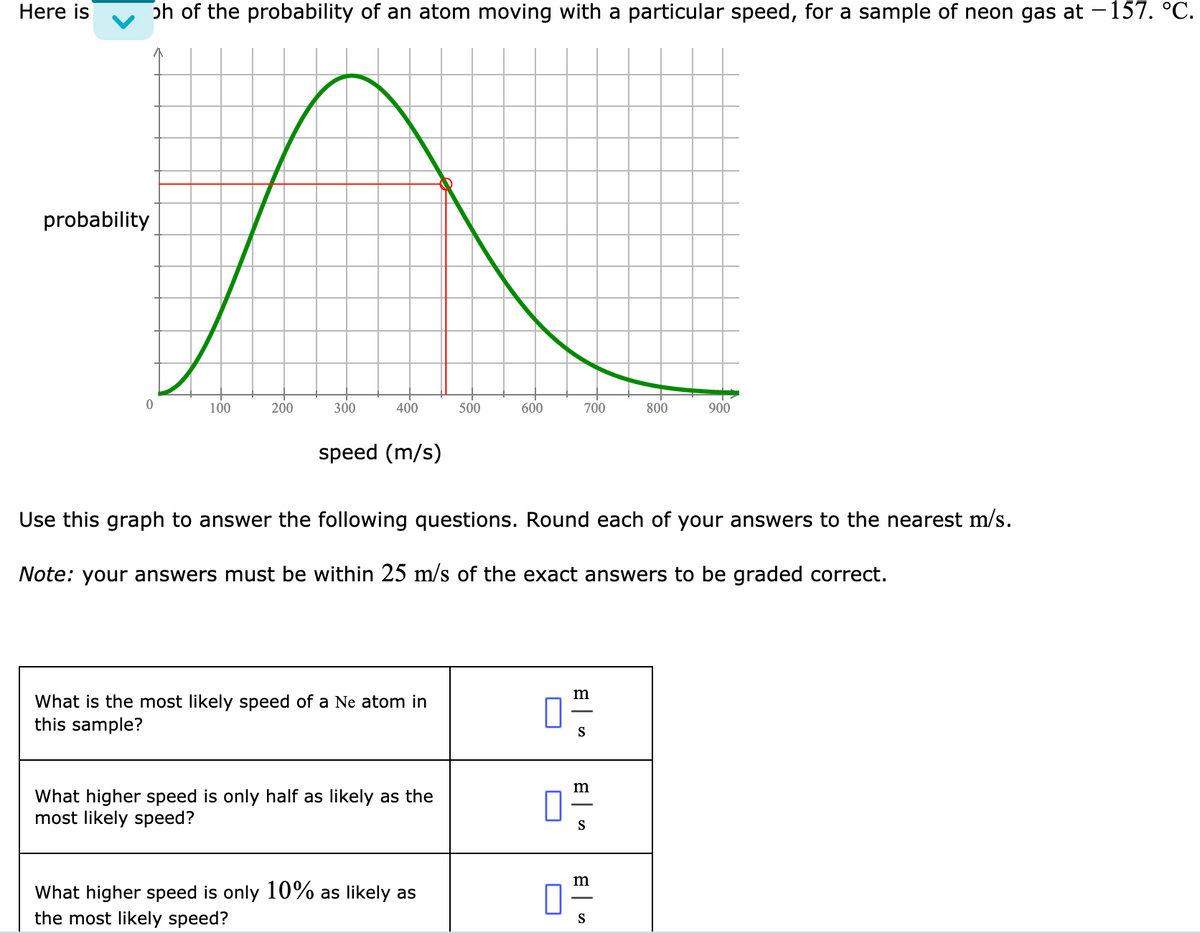
Transcribed Image Text:Here is
ph of the probability of an atom moving with a particular speed, for a sample of neon gas at -157. °C.
probability
100
200
300
400
500
600
700
800
900
speed (m/s)
Use this graph to answer the following questions. Round each of your answers to the nearest m/s.
Note: your answers must be within 25 m/s of the exact answers to be graded correct.
m
What is the most likely speed of a Ne atom in
this sample?
S
What higher speed is only half as likely as the
most likely speed?
S
m
What higher speed is only 10% as likely as
the most likely speed?
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning