highlighted reactant reaction Bronsted-Lowry Bronsted-Lowry neither acid base HSO (aq) + H,O"(aq) → H,SO,(aq) + H,0(1) H,SO (aq)+ H,O(1) HSO,(aq) + H,O" (aq) HSO (a) + H,0"(aq) - H, SO,(aq) + H,0(1) H,SO(aq) + H,0() HSO (aq) + H,0 (aq)
highlighted reactant reaction Bronsted-Lowry Bronsted-Lowry neither acid base HSO (aq) + H,O"(aq) → H,SO,(aq) + H,0(1) H,SO (aq)+ H,O(1) HSO,(aq) + H,O" (aq) HSO (a) + H,0"(aq) - H, SO,(aq) + H,0(1) H,SO(aq) + H,0() HSO (aq) + H,0 (aq)
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter17: Acid-base(proton Transfer) Reactions
Section: Chapter Questions
Problem 17.2TC
Related questions
Question
100%
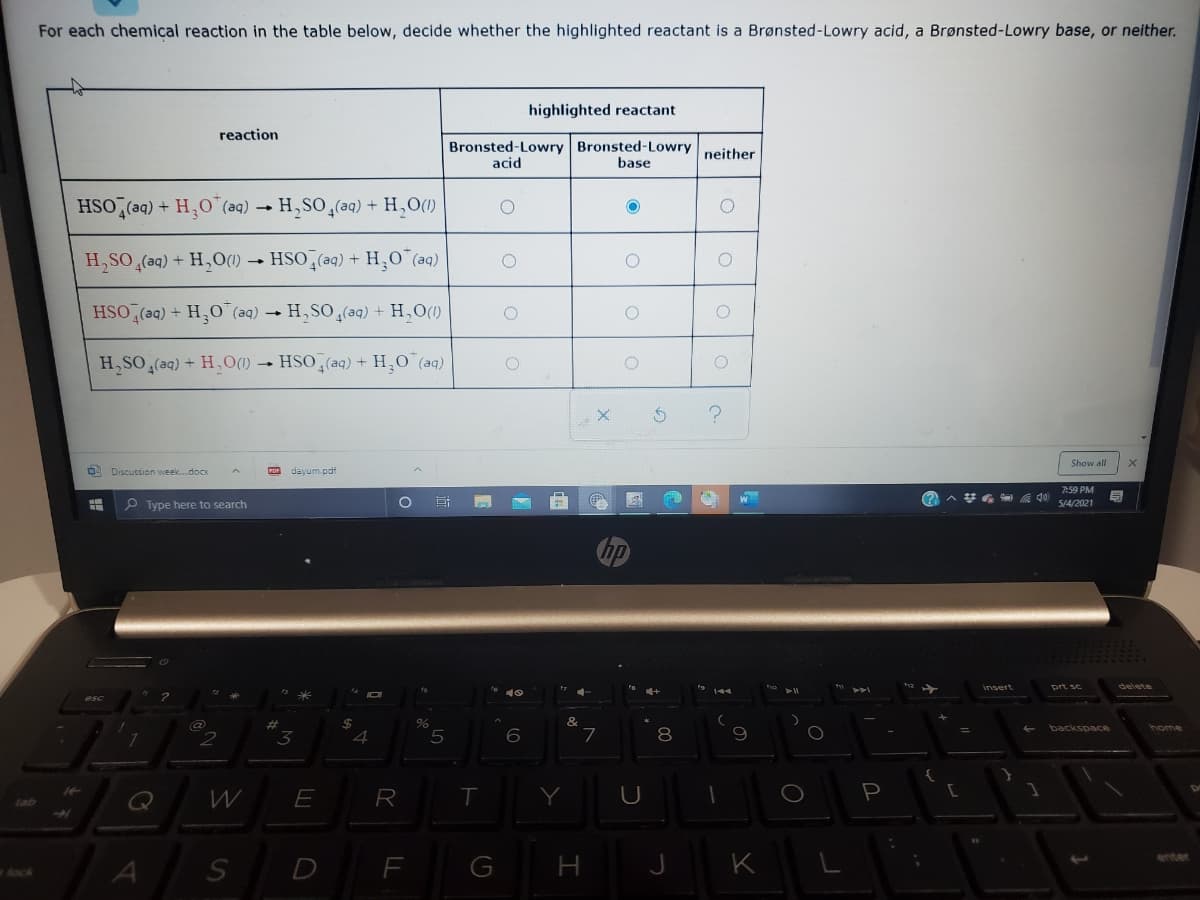
Transcribed Image Text:For each chemical reaction in the table below, decide whether the highlighted reactant is a Brønsted-Lowry acid, a Brønsted-Lowry base, or neither.
highlighted reactant
reaction
Bronsted-Lowry Bronsted-Lowry neither
acid
base
HSO,(aq) + H,0"(aq) → H,SO,(aq) + H,O(1)
H,SO (aq) + H,O(1)
HSO (aq) + H,0 (aq)
HSO (aq) + HO"(aq) → H, SO(aq) + H,O(1)
H,SO (aq) + H,O(1) → HSO,(aq) + H,0 (aq)
Show all
O Discussion week.docx
dayum.pdf
7:59 PM
司
P Type here to search
5/4/2021
hp
insert
prt se
చ
&
%24
4
backspace
home
8.
R
tab
K
enter
D
F
G
H
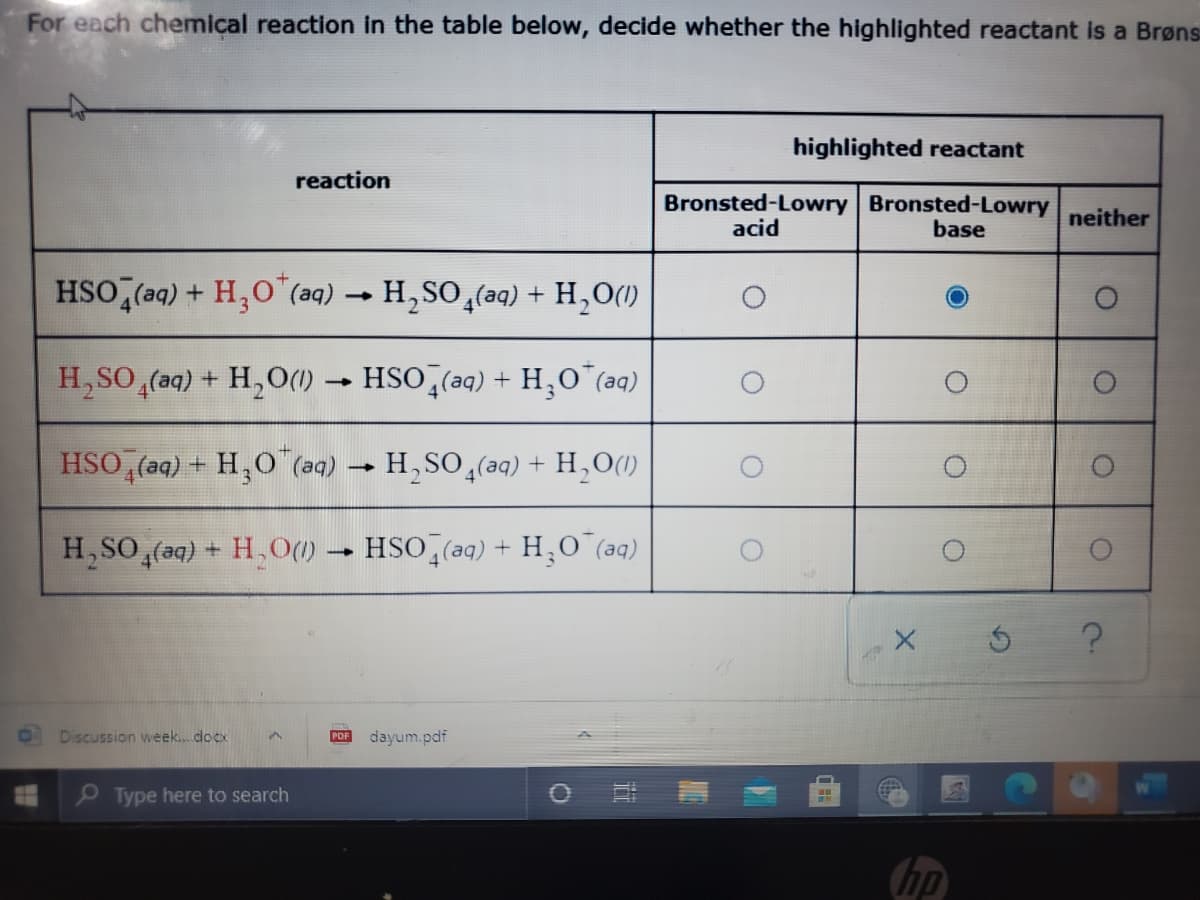
Transcribed Image Text:For each chemical reaction in the table below, decide whether the highlighted reactant is a Brøns
highlighted reactant
reaction
Bronsted-Lowry Bronsted-Lowry
neither
acid
base
HSO,(aq) + H,0"(aq) → H,SO,(aq) + H,O()
H,SO,(aq) + H,O() -
HSO (aq) + H,O"(aq)
HSO, (aq) + H,O (aq)
H,SO (aq) + H,O(1)
H, SO (aq) + H, O()
HSO (aq) + H,O (aq)
Discussion week.docx
dayum.pdf
PDF
O Type here to search
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning