HO. но- OH O-R 26. What is the purpose of achieving the transition state of the substrate in the active site of the enzyme? A. It allows ionic charges to form. B. It leads to formation of product. C. It relieves strain in covalent bonds. D. It keeps the substrate activated.
HO. но- OH O-R 26. What is the purpose of achieving the transition state of the substrate in the active site of the enzyme? A. It allows ionic charges to form. B. It leads to formation of product. C. It relieves strain in covalent bonds. D. It keeps the substrate activated.
Biology: The Dynamic Science (MindTap Course List)
4th Edition
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Chapter6: Energy, Enzymes, And Biological Reactions
Section: Chapter Questions
Problem 6TYK: Which of the following methods is not used by enzymes to increase the rate of reactions? a. covalent...
Related questions
Question
8
Transcribed Image Text:HO.
но-
OH
O-R
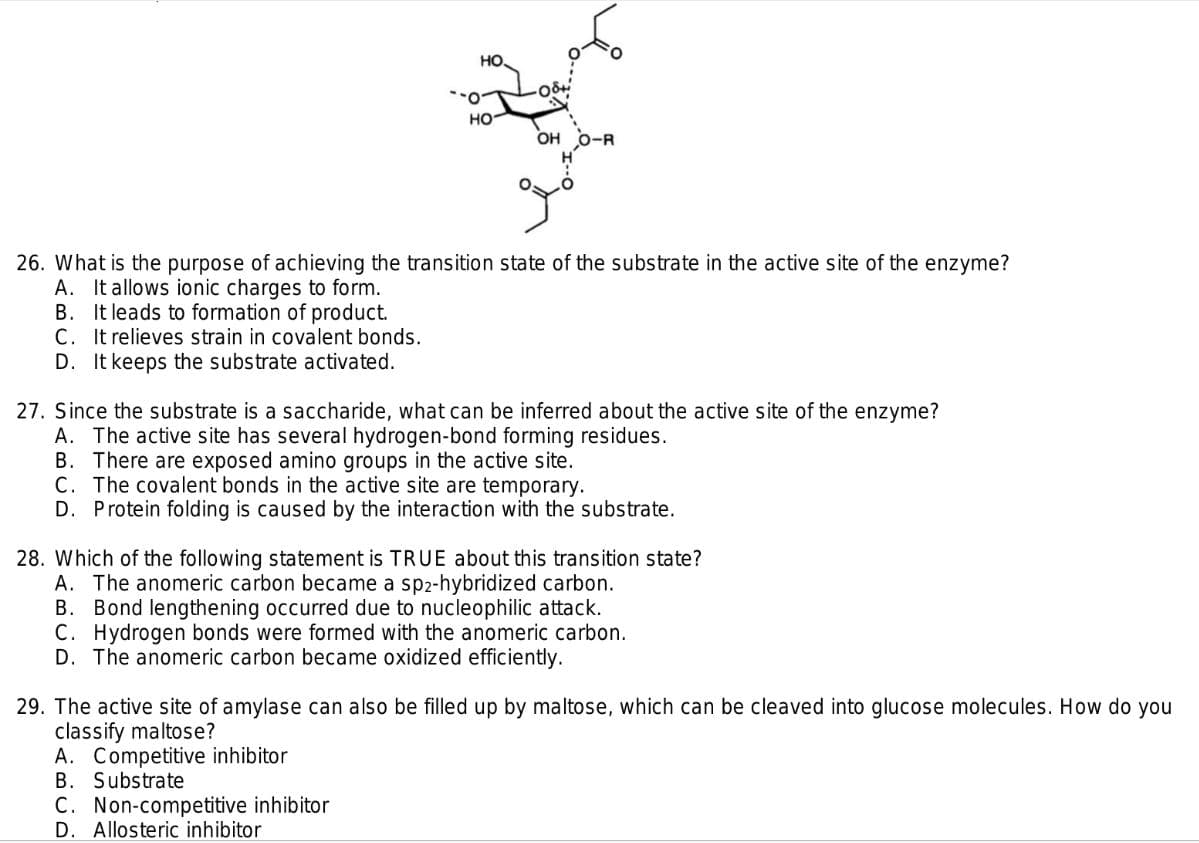
26. What is the purpose of achieving the transition state of the substrate in the active site of the enzyme?
A. It allows ionic charges to form.
B. It leads to formation of product.
C. It relieves strain in covalent bonds.
D. It keeps the substrate activated.
27. Since the substrate is a saccharide, what can be inferred about the active site of the enzyme?
A. The active site has several hydrogen-bond forming residues.
B. There are exposed amino groups in the active site.
C. The covalent bonds in the active site are temporary.
D. Protein folding is caused by the interaction with the substrate.
28. Which of the following statement is TRUE about this transition state?
A. The anomeric carbon became a sp2-hybridized carbon.
B. Bond lengthening occurred due to nucleophilic attack.
C. Hydrogen bonds were formed with the anomeric carbon.
D. The anomeric carbon became oxidized efficiently.
29. The active site of amylase can also be filled up by maltose, which can be cleaved into glucose molecules. How do you
classify maltose?
A. Competitive inhibitor
B. Substrate
C. Non-competitive inhibitor
D. Allosteric inhibitor
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Recommended textbooks for you

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning