Chapter3: Using Spreadsheets In Analytical Chemistry
Section: Chapter Questions
Problem 3.4QAP
Related questions
Question
I just only want to have an answer of the D option related with the 3.reaction?
How are we going to calculate the LnKp value of 3.reaction?(((not Kp but LnKp)) and what is the value?
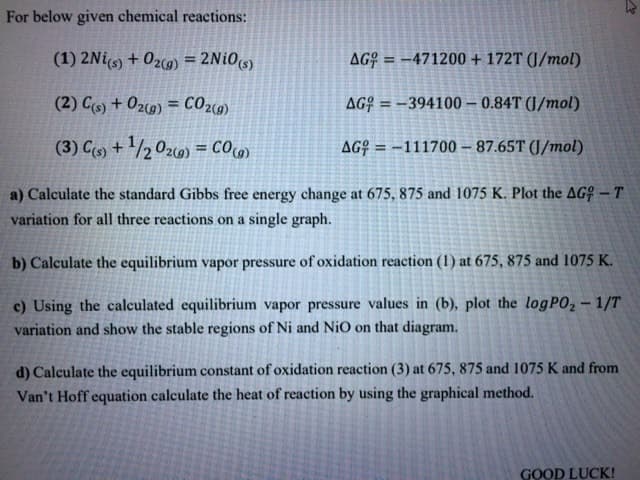
Transcribed Image Text:For below given chemical reactions:
(1) 2Nis) + 02cg) = 2NiOs)
AG? = -471200 + 172T (J/mol)
%3!
%3D
(2) Cs) + O2c9) = CO29)
AG¢ = –394100 – 0.84T (J/mol)
%3D
(3) C + /2 0z9) = C09)
AG¢ = -111700 – 87.65T (J/mol)
a) Calculate the standard Gibbs free energy change at 675, 875 and 1075 K. Plot the AG – T
variation for all three reactions on a single graph.
b) Calculate the equilibrium vapor pressure of oxidation reaction (1) at 675, 875 and 1075 K.
c) Using the calculated equilibrium vapor pressure values in (b), plot the logP02 – 1/T
variation and show the stable regions of Ni and NiO on that diagram.
d) Calculate the equilibrium constant of oxidation reaction (3) at 675, 875 and 1075 K and from
Van't Hoff equation calculate the heat of reaction by using the graphical method.
GOOD LUCK!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

