How can you calculate the concentration of a solution with UV-vis spectroscopy if you do NOT know the molar absorption coefficient? (a) Add blue dye to the sample and then subtract the blank sample's absorbance. (b) Use Beer's Law by assigning the molar absorption coefficient a value of 1 (e = 1). (c) Create a calibration curve which is a plot of absorbance vs concentration. (d) Calculate the rate constant. (e) Divide the measured absorbance by the product of l´e.
How can you calculate the concentration of a solution with UV-vis spectroscopy if you do NOT know the molar absorption coefficient? (a) Add blue dye to the sample and then subtract the blank sample's absorbance. (b) Use Beer's Law by assigning the molar absorption coefficient a value of 1 (e = 1). (c) Create a calibration curve which is a plot of absorbance vs concentration. (d) Calculate the rate constant. (e) Divide the measured absorbance by the product of l´e.
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.1QAP
Related questions
Question
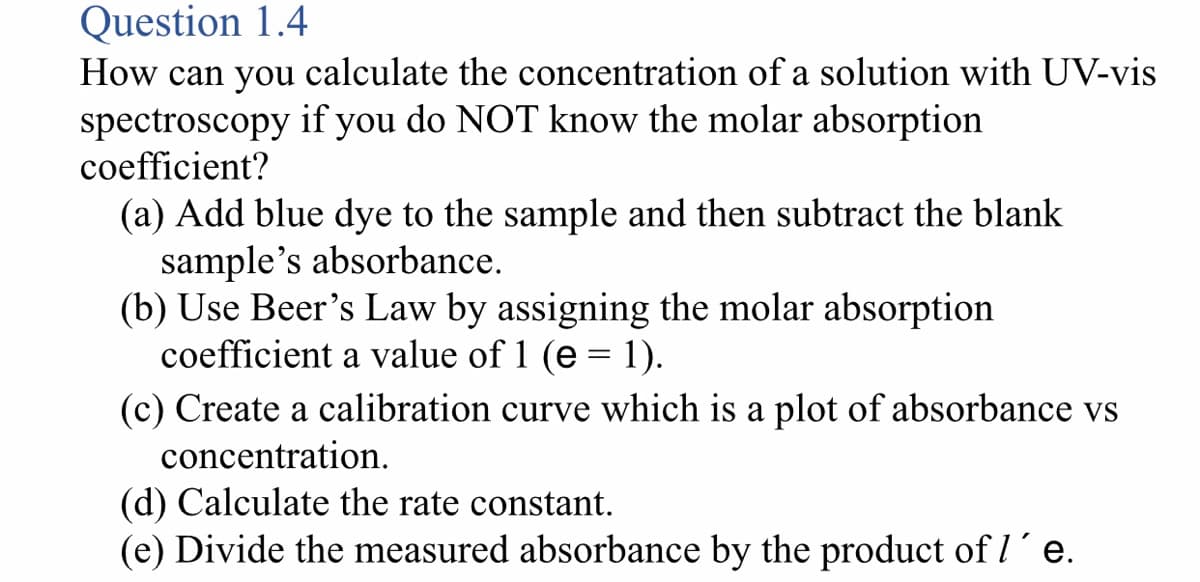
Transcribed Image Text:Question 1.4
How can you calculate the concentration of a solution with UV-vis
spectroscopy if you do NOT know the molar absorption
coefficient?
(a) Add blue dye to the sample and then subtract the blank
sample's absorbance.
(b) Use Beer's Law by assigning the molar absorption
coefficient a value of 1 (e = 1).
(c) Create a calibration curve which is a plot of absorbance vs
concentration.
(d) Calculate the rate constant.
(e) Divide the measured absorbance by the product of I´e.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning