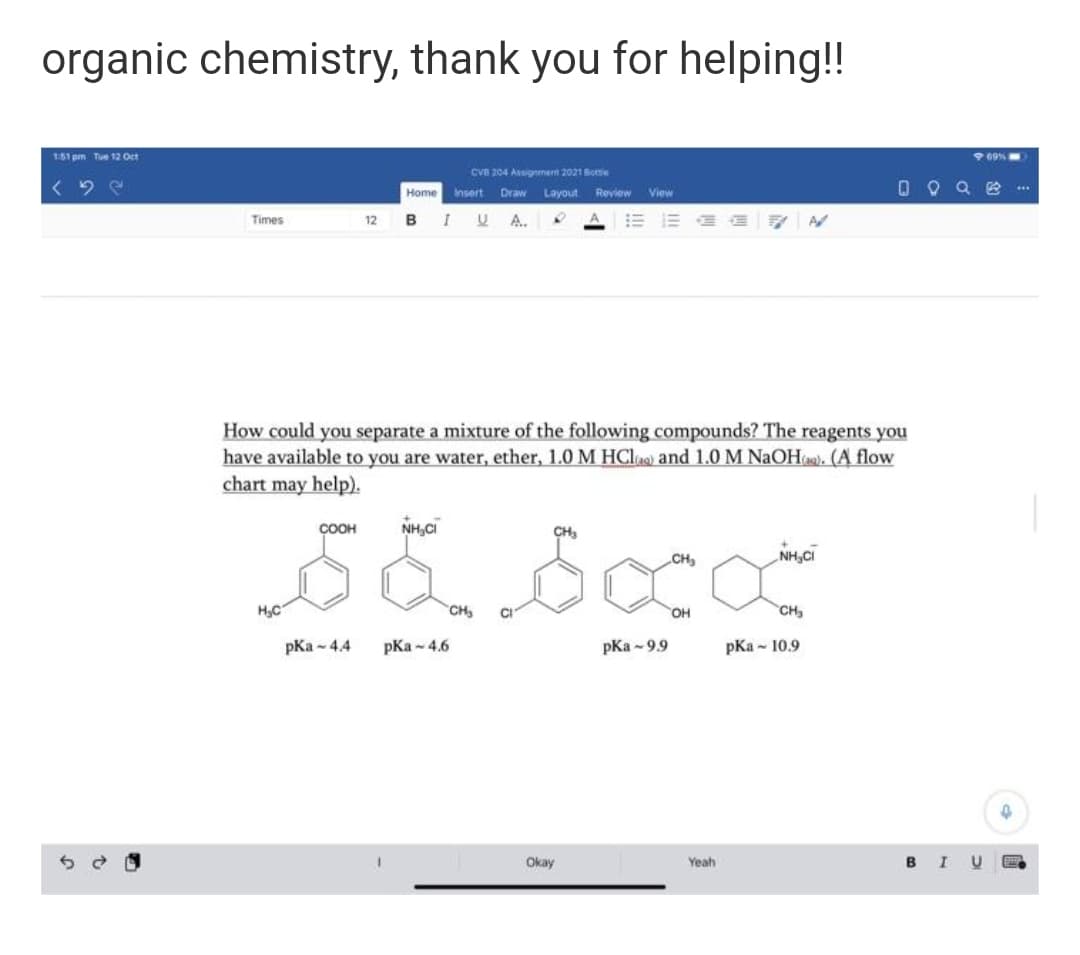
How could you separate a mixture of the following compounds? The reagents you have available to you are water, ether, 1.0 M HCla) and 1.0 M NAOH). (A flow chart may help). ÇOOH NH,CI CH, CH, NH,CI CH OH CH pKa - 44 pKa - 4.6 pKa - 9.9 pKa - 10.9
Q: Some 6 M hydrochloric acid is added to an unknown. Bubbles appear. Which ion, from this experiment,…
A: In the given reaction 6 M hydrochloric acid reacts with an unknown, and bubbles appear. We know that…
Q: 45. Which of these chromates has the lowest molar solubility? a. BaCrO4 (Ksp=2.1x10-10) b. PbCrO4…
A:
Q: Post-lab Question #3: What is the pH of the solution that is 0.75 M in sodium acetate and 0.50 M in…
A:
Q: 10-14. The Zn in a 0.7556-g sample of foot powder was titrated with 21.27 mL of 0.01645 M EDTA.…
A: Titration is a method of determining the amount of chemical species present by reacting it with a…
Q: 100 mL of 1.0 M formic acid (HCOOH) is titrated with 1.0 M sodium 9. hydroxide (NaOH). The titration…
A: a.) We would use the fact that at half equivalance point , pH = pKa . b.) As pKa is point of half…
Q: Consider the following information: Ksp(BaSO4) = 1.1 x 10-10 Ksp(CaSO4) = 4.9 x 10-5 Ksp(PBSO4) =…
A:
Q: Which of the following compounds has the lowest solubility in mol/L in water? a. Al(OH)3 Ksp…
A: The solution is given below -
Q: The pH of a river was determined to be 8 and its CO32- concentration was 2.5 x 10-5 M. Given the…
A: Concentration of CO3-2 = [ 2.5 × 10-5 ] Value of dissociation constant = 4.7 × 10-11 pH of river =…
Q: Which indicator should you use for a titration between a strong acid and a strong base? A.)…
A: Titration between strong acid and strong base ,equivalence point arrises at around 7.0 Thus that…
Q: ksp = 11.8 x 10-11 at 25 degree C. A saturated solution has a pH of 10.5 and the Mg2+…
A: At pH 9 the OH- ion concentration is, pOH = 14 -pH = 14- 9 = 5 [OH-] = 10(-5) [OH-]…
Q: A weak acid has KĄ = 4.31 x 10-4. What is the pKĄ of this acid? Report your answer to at least 1…
A:
Q: A soltion is made by the addition of 40 ml of 40 mM NaO to 40 of 30 mM phoshork acd What is the…
A: Given: Concentration of NaOH = 40 mM = 0.040 M (Since 1 M = 1000 mM)…
Q: Which of the following comninations will result in a precipitate? Pb2+ + Cl- bBa2+ + CO3 2- Fe3+…
A: To know the solubility of salt, some solubility rules are given. For example, salts of Na+ , K+ ,…
Q: KOt-Bu Br OH HCI
A: The required reactions are
Q: can you help me calculate the percent error for my pKa? my pKa = 6.79 my Ka = 1.62 x 10^-7 the…
A: The experimental value of pKa of NaHSO3 = 6.79 NaHSO3 is a single deprotonated form of H2SO3 whose…
Q: pka E
A:
Q: 7. For aldehydes, a common quantification method is: A) Polarimetry B) Iodometry (back titration)…
A: Aldehyde is represented as - R - CHO, where R = Alkyl group In presence of an oxidising agent,…
Q: 2+ hat will the concentration of Mg?t
A:
Q: A 0.9182 g sample of CaBr2 is dissolved in enough water to give 500. mL of solution. What is the…
A: Solution: Number of moles present in a solution give the ratio of number of moles of solute to the…
Q: What are the predicted products when Ca(OH)2 dissociates in water? Group of answer choices Ca2+ +…
A: We have to predict products when Ca(OH)2 dissociates in water as follows in step 2:
Q: In 6.0 M NaOH, M(OH)3 has a solubility of 1.0 M due to the formation of the [M(0H)4]¯ ion. Calculate…
A: Interpretation - To calculate the Ksp of M(OH)3 when In a 6M NaOH , M(OH)3 has a solubility of 1M…
Q: (i) (ii) (iii) (iv) dissociation constant of [Cu(NH 3)4]2+ is x × 10-12, then value of 'x' is Cu2+ +…
A:
Q: 6. Find the pH of a 1.00-L aqueous solution prepared with 14.23 g of tris (FM 121.00) plus 5.67 g of…
A:
Q: Given that the solubility of PbCrO4(s) (323.2g/mol) is 4.5 x 10-5g/L, calculate the Ksp of PbCrO4…
A:
Q: calculate the pH of a 0.5L solution prepared with 0.05 M acetic acid (pK = 4.76) before and after…
A:
Q: What is the Ksp expression for this dissolving reaction? NazPO4(s) = 3Na*(aq) + PO43(aq) -3, O A.…
A: Ksp expression is written as products of concentration of ions raise to the power the coefficients .
Q: A 0.21-mol sample of a diprotic acid, H 2A, is dissolved in 250 mL of water. The Ka1 of this acid is…
A: we have to calculate the concentration of A2-
Q: Which one of the following indicators would be best to use when titrating 0.1M morphine (Kb = 1.6 ×…
A: pH is a measure of hydrogen ion concentration in solution and it measures acidity or alkalinity of a…
Q: mix 500 ml of 0.1 M HCl solution with 300 ml of 2 M Ba (OH) 2 solution a) indicate the environment…
A:
Q: Find Ka and pka for the unknown weak acid. Calculate [A-]eq and [HA]eq to help. Given: HA + H2O…
A: Answer:- This question is answered by using the simple concept of calculation of Ka and pKa value…
Q: What is the H 3O + concentration in 0.0055 M Ba(OH) 2(aq) at 25 °C? ( K w = 1.01 × 10 –14)? a.…
A: Kw = 1.01e-14 Concentration of barium hydroxide = 0.0055 M Concentration of H3O+ ion = ?
Q: A weak acid. What is the pHpH of a 0.1 M0.1 M solution of acetic acid (pKa=4.75)?(pK, = 4.75)? %3D
A: Given that, Concentration of acetic acid = 0.1 M pKa of acetic acid = 4.75 pKa is given by: pKa =…
Q: Identify the structure(s) observed at each point (a-c) indicated along the titration curve. Point…
A: We have match the curves to appropriate compound.
Q: A solution of hydrazoic acid, HN3 (43.03 mg/mmol), a colorless, volatile, and explosive liquid, was…
A:
Q: how many mLs of 0.125 M Ba(OH)2 would be required to completely nuetralize 75.0 mL of 0.845 M HCI?…
A: Given Molarity of Ba(OH)2 = 0.125 M = 0.125 mol/L Volume of…
Q: Ascorbic Acid Content in Vitamin C Supplements Part 3. Titration of Unknown Ascorbic Acid Solution…
A: Answer: This question is based on the stoichiometric calculation where first of all we have to find…
Q: C. Standardization of HCI titrant 1. Dry 1.5 to 2.0 g pure NazCOs in a glass weighing bottle at 150…
A: Given: molar mass of Na2CO3 = 105.988 g/mol
Q: Periodic acid, HIO4, is an important oxidizing agent and a moderately strong acid. In a 0.10 M…
A:
Q: How many milliliters of 1.33 M KOH should be added to 100 mL of solution containing 10.0 g of…
A: Mechanism of histidine and their respective pH is shown below:
Q: Consider the following data: HX ↔ X− + H+HX ↔ X- + H+ Ka =…
A: Given reactions HX ↔ X− + H+ Ka = 6.83×10-6 H2A ↔ HA− + H+ Ka = 6.85×10-8…
Q: Calculate the Ka of HA given A−(aq)+H2O(l)⇌OH−(aq)+HA(aq) Kb = 9.60×10-7
A: Given reaction : A-(aq.) + H2O(l) ⇌ OH-(aq.) + HA(aq.) Kb = 9.60×10-7 We can use the relation…
Q: 4. What effect would the following errors have on the final value of Ka determined from this…
A: Acid dissociation constant ( Ka ): It is the ratio of the concentration of Product to that of the…
Q: for a 0.10 M solution of a weak acid, HA, with pKa=10, which of the following is true? a. [HA]=[A-]…
A: Weak acids undergo partial dissociation in water.
Q: Tris has a pKa of 8.1 at 25°C (Tris MW=121.1 g/mol)(Tris-HCl MW=157.6 g/mol). We need 250 mL of 0.1…
A: Buffer is a solution which tends to resist the change in its pH even after addition of acid or base…
Q: Show your working clearly Lead chloride has ksp = 1.7 x10-5 at room temperature. Calculate its…
A: We have to use solubility product and common ion effect concept for this question
Q: What is the Main Content uffer that is 0.452 M HF and 0.404 M LiF? The K, for HF is 3.5 x 10-4. Your…
A: Buffer solution :- A solution which resists the change in its pH value by the addition of small…
Q: 9 what is the PH of a 2.5 M solutien ef CH, NH, CI ? CHgNHz pkb = 3.35) %3D
A:
Q: What is the molar solubility of a hypothetical compound AB in a solution containing 19.92 M B- given…
A: Given: Concentration of B-, [B- ] = 19.92 M
Q: Clearly show your working. Lead chloride has ksp = 1.7 x10-5 at room temperature. (i)Calculate its…
A:
Q: Compute for the pKa of benzoic acid (Ka = 6.3 × 10-5).
A: Given acid dissociation constant (Ka) of benzoic acid = 6.3 × 10-5 acid dissociation constant (Ka)…
M¹
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- Alcohols can undergo a lot of different reaction mechanims. If the alcohol group (OH) is attaached to an aromatic core, how will the chemistry change as compared to a typical alkyl alcohol? A) The OH group will become more polarised and more nucleophilic. B) The OH group will become more susceptible to oxidation C) The OH group will become more polarised and therefore basic D) The OH group will become more polarised and therefore acidic.C13b This is for my reviewer please help me with the step by step solution and answer, thank youI have this task in organic chemistry (book: Brown's introduction to organic chemisty, global edition). Task 10:42. In (a) I have to tell what the funcion of K2CO3 is in step 1. Is it that CO32- take the hydrogen atom in 1-napthol? Will it then be a SN2 mechanism? In (b) I have to name the amine used in step 2 to form Propanolol. But I can't really find out how to come up with an amine that will make that reaction. Here are two pictures of the task:
- So I cant see any of the answers on section 4.41 D, F, G In the Organic Chemistry: Principles And Mechanisms (second Edition) 2nd Edition Textbook. Would you be able to just send me the answers and images. I have already done them and I am practicing for class but I dont know if I am correct.Please help me with the organic chemistry question below. The solutions are linked, as it is one question with 2 parts: 1. A. Draw a molecular orbital diagram for (2E,4E)hexa-2,4-diene and ethene below. Make sure to label orbitals, HOMO, LUMO, nodes, antibonding/bonding, and fill correctly with electrons. 1. B. Use the orbitals drawn in the previous problem to determine if a cycloadditon would occur between the two compounds mentioned and if so, would it be suprafacial or antarafacial. Be sure to specify which orbitals you are using and use those orbitals to demonstrate your answer.For the Organic Chemistry topic of R and S, to make the structure R, does the prioritizing number have to be in order from 1 -> 2 -> 3 clockwise or can it be clockwise from 3 -> 2 -> 1?
- 3) Enols are an important class of molecules in organic chemistry, which are utilized for theirability to form carbon-carbon bonds. We will learn about their unique reactivity much later inthe course. Shown below is an enol on the left, whereas the molecule on the right is classifiedas an allylic alcohol. a) Please explain why the structure on the right IS NOT a resonance structure of the enol on theleft. A short statement or illustration is fine, be sure that your rationale is clear. b) Draw the correct resonance structure that can be produced from the enol on the left. Besure to include arrows to indicate the movement of electrons in the left-hand structure andindicate charges in the resulting structure. c) What is the hybridization of the carbon indicated with the red arrow?1. Write the a) Lewis dot formula, b) line angle formula and of the following molecules: A. HCN B. NH3 2. What is the difference between homolysis vs. heterolysis. Show by giving an example.! (plz give proper explanation)
- Uemura and coworkers studied a time dependent Diels-Alder reaction which first formed the endo product as the major organic product and with time produced the exo product (J. Org. Chem. 2018, 83, 9300−9304). Show the endo and exo product for the reaction below. Which is the thermodynamic product and which is the kinetic product? Explain your reasoning.Organic Chemistry 1 Absolute Configuration S or R (CIP) Explain all of the steps clearly and logically. Do not just draw a picture. Clearly explain your answer in a step-by-step fashion. Here is the instructor's solution (answer/explanation) to the problem. Please read the professor's solution carefully and explain it here. I have the solutions since the assignment has been turned in. I want to understand why the answer key is correct, and I need a more detailed explanation than the answer key.How many atoms of each hybridization state are there (sp,sp2, sp3)?What would be the result after the shown molecule is carried through the 4 step sequence? Show each intermediate and stereochemistry 1. 1eq. MeLi, then H2O workup 2.TsCl/pyr 3.NaOtBu/BuOH 4.OsO4/NaHSO3 And What is the relationship between the isomeric end products from them?

