How did Rutherford interpret the fact that a few of the a particles were deflected at very large angles? O The a particles were striking the negatively charged electrons in the gold atoms. O The a particles were striking the neutrally charged neutrons in the gold atoms. O The a particles were striking the small, positively charged nucleus of the gold atoms. What differences would Rutherford have observed if he used aluminum foil instead of gold foil? O All of the a particles would have passed through the aluminum foil unobstructed, and no particles would have deflected at a large angle. O Less of the a particles would have passed through the aluminum foil unobstructed, and more would have been deflected at a large angle. O More of the a particles would have passed through the aluminum foil unobstructed, and less would have been deflected at a large angle. O None of the a particles would have passed through the aluminum foil, and all of them would have deflected at a large angle.
How did Rutherford interpret the fact that a few of the a particles were deflected at very large angles? O The a particles were striking the negatively charged electrons in the gold atoms. O The a particles were striking the neutrally charged neutrons in the gold atoms. O The a particles were striking the small, positively charged nucleus of the gold atoms. What differences would Rutherford have observed if he used aluminum foil instead of gold foil? O All of the a particles would have passed through the aluminum foil unobstructed, and no particles would have deflected at a large angle. O Less of the a particles would have passed through the aluminum foil unobstructed, and more would have been deflected at a large angle. O More of the a particles would have passed through the aluminum foil unobstructed, and less would have been deflected at a large angle. O None of the a particles would have passed through the aluminum foil, and all of them would have deflected at a large angle.
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
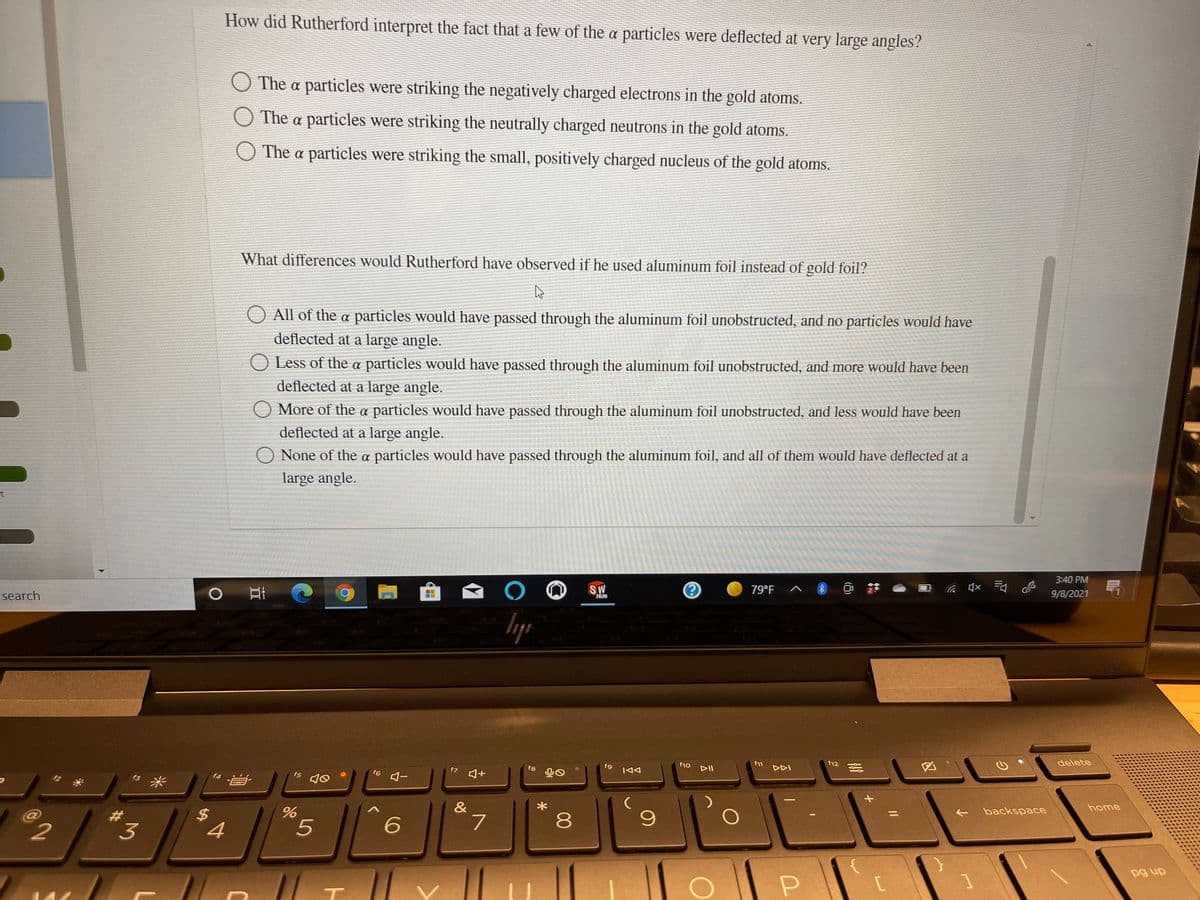
Transcribed Image Text:How did Rutherford interpret the fact that a few of the a particles were deflected at very large angles?
O The a particles were striking the negatively charged electrons in the gold atoms.
O The a particles were striking the neutrally charged neutrons in the gold atoms.
The a particles were striking the small, positively charged nucleus of the gold atoms.
What differences would Rutherford have observed if he used aluminum foil instead of gold foil?
All of the a particles would have passed through the aluminum foil unobstructed, and no particles would have
deflected at a large angle.
O Less of the a particles would have passed through the aluminum foil unobstructed, and more would have been
deflected at a large angle.
More of the a particles would have passed through the aluminum foil unobstructed, and less would have been
deflected at a large angle.
O None of the a particles would have passed through the aluminum foil, and all of them would have deflected at a
large angle.
3:40 PM
search
SW
79°F
9/8/2021
2020
lyp
f10
f11
f12
delete
f6 d-
f7
f8
f9
144
DII
DDI
f5
f3
&
home
backspace
%24
4
%23
7
2
3
pg up
00
5
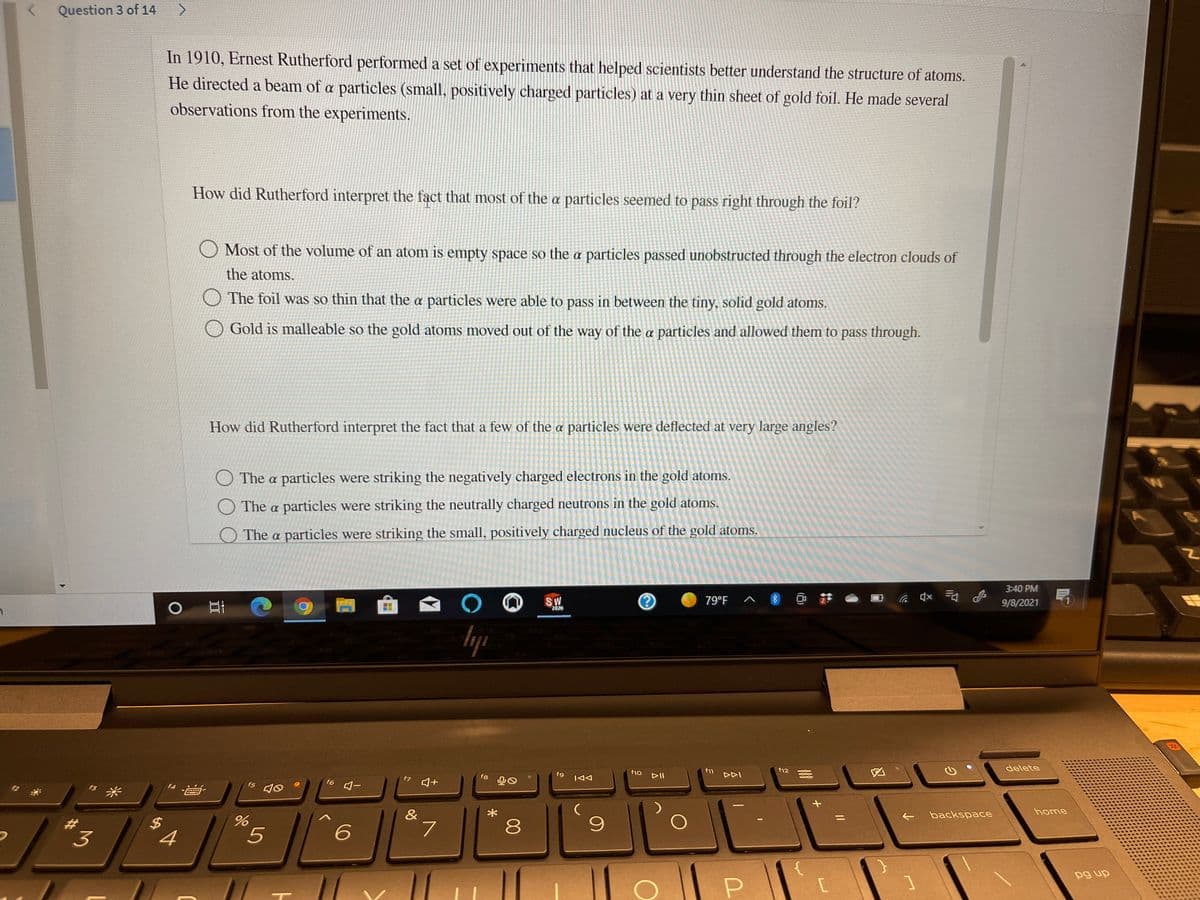
Transcribed Image Text:Question 3 of 14
In 1910, Ernest Rutherford performed a set of experiments that helped scientists better understand the structure of atoms.
He directed a beam of a particles (small, positively charged particles) at a very thin sheet of gold foil. He made several
observations from the experiments.
How did Rutherford interpret the fact that most of the a particles seemed to pass right through the foil?
O Most of the volume of an atom is empty space so the a particles passed unobstructed through the electron clouds of
the atoms.
The foil was so thin that the a particles were able to pass in between the tiny, solid gold atoms.
Gold is malleable so the gold atoms moved out of the way of the a particles and allowed them to pass through.
How did Rutherford interpret the fact that a few of the a particles were deflected at very large angles?
The a particles were striking the negatively charged electrons in the gold atoms.
O The a particles were striking the neutrally charged neutrons in the gold atoms.
O The a particles were striking the small, positively charged nucleus of the gold atoms.
3:40 PM
SW
79°F
9/8/2021
2020
f12
delete
f10
f11
fg
DII
DDI
f7 +
f8
144
f6 -
f5
&
backspace
home
%23
3
$4
4.
L.
pg up
P
中。
心)
00
%24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 2 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON