Malin just discovered a new protein, Malinase and for it not to unfold and lose its function, he realized that the protein needs to be constantly immersed in a pH = 11.90 solution. He is therefore interested in preparing a 50.0 mL 0.080 M pH = 11.90 buffer to contain the protein. He listed down the acid solutions available to his disposal, and also looked for their acid dissociation constant (Ka) values. ACID Ка Каз Кa N/A N/A 4.00 x 10* 2.00 M NITROUS ACID (HNO:) 2.00 M ACETIC ACID (CH,COOH) 2.00 M ASCORBIC ACID (H;Asc) 2.00 M PHOSPHORIC ACID (H;POA) 7.52 x 10 6.23 x 10 4.28 x 103 N/A 1.76 x 10 N/A 7.90 x 10 1.60 x 102 N/A A. Identify which weak acid system is best to use in preparing the buffer? Why? В. Identify the species which will make up the buffer system? C. prepare the buffer Calculate the amount (in mmoles) of each of the components necessary to D. Indicate how Malin is supposed to prepare the buffer from 2.00 M of the acid and/or 4.00 M N2OH. E. Calculate the resulting pH if 5 drops (1 ml = 20 drops) 0.500 M HCI was accidentally added to the buffer.
Malin just discovered a new protein, Malinase and for it not to unfold and lose its function, he realized that the protein needs to be constantly immersed in a pH = 11.90 solution. He is therefore interested in preparing a 50.0 mL 0.080 M pH = 11.90 buffer to contain the protein. He listed down the acid solutions available to his disposal, and also looked for their acid dissociation constant (Ka) values. ACID Ка Каз Кa N/A N/A 4.00 x 10* 2.00 M NITROUS ACID (HNO:) 2.00 M ACETIC ACID (CH,COOH) 2.00 M ASCORBIC ACID (H;Asc) 2.00 M PHOSPHORIC ACID (H;POA) 7.52 x 10 6.23 x 10 4.28 x 103 N/A 1.76 x 10 N/A 7.90 x 10 1.60 x 102 N/A A. Identify which weak acid system is best to use in preparing the buffer? Why? В. Identify the species which will make up the buffer system? C. prepare the buffer Calculate the amount (in mmoles) of each of the components necessary to D. Indicate how Malin is supposed to prepare the buffer from 2.00 M of the acid and/or 4.00 M N2OH. E. Calculate the resulting pH if 5 drops (1 ml = 20 drops) 0.500 M HCI was accidentally added to the buffer.
Chapter26: Biomolecules: Amino Acids, Peptides, And Proteins
Section26.SE: Something Extra
Problem 50AP: The -helical parts of myoglobin and other proteins stop whenever a proline residue is encountered in...
Related questions
Question
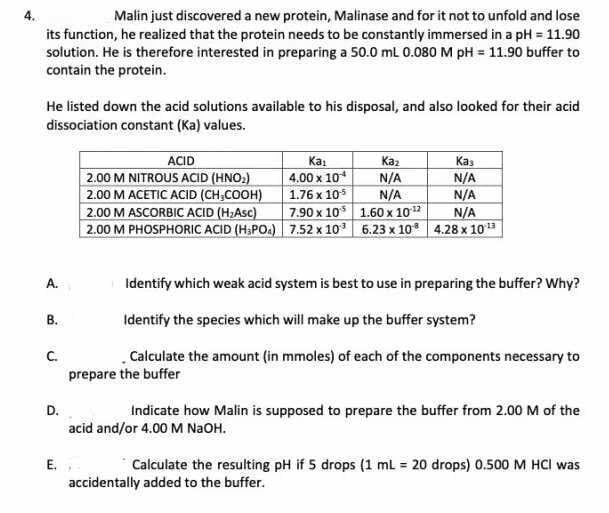
Transcribed Image Text:Malin just discovered a new protein, Malinase and for it not to unfold and lose
its function, he realized that the protein needs to be constantly immersed in a pH = 11.90
solution. He is therefore interested in preparing a 50.0 mL 0.080 M pH = 11.90 buffer to
contain the protein.
He listed down the acid solutions available to his disposal, and also looked for their acid
dissociation constant (Ka) values.
ACID
2.00 M NITROUS ACID (HNO:)
2.00 M ACETIC ACID (CH,COOH)
2.00 M ASCORBIC ACID (H:Asc)
Ka:
4.00 x 10
N/A
1.76 x 105
N/A
7.90 x 10 1.60 x 102
Kaz
Каз
N/A
N/A
N/A
2.00 M PHOSPHORIC ACID (H:PO4) 7.52 x 10 6.23 x 10 4.28 x 103
A.
Identify which weak acid system is best to use in preparing the buffer? Why?
В.
Identify the species which will make up the buffer system?
C.
prepare the buffer
. Calculate the amount (in mmoles) of each of the components necessary to
D.
Indicate how Malin is supposed to prepare the buffer from 2.00 M of the
acid and/or 4.00 M N2OH.
E.
Calculate the resulting pH if 5 drops (1 ml = 20 drops) 0.500 M HCI was
accidentally added to the buffer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
D. How is he supposed to prepare the buffer from 2.00 M of the acid and/or 4.00 M N2OH
E. Calculate the resulting pH if 5 drops (1 ml = 20 drops) 0.500 M HCI was accidentally added to the buffer.
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning