A solution is saturated at 25 g per 100 g of solution; this solution has 22 grams in 100 g of solution. unsaturated It contains as much solute molecules as the solvent can hold at a given temperature. Crystallization occurs when a small amount of solute is added. Is a mixture. supersaturated A solution is saturated at 25 g per 100g of solution; this solution has 25 grams in 100 g of solution. It contains more solute molecules than usually can dissolve. Is cloudy. A solution saturated at 25 g per 100 g of solution; this solution has 31 grams in 100 g of solution.
A solution is saturated at 25 g per 100 g of solution; this solution has 22 grams in 100 g of solution. unsaturated It contains as much solute molecules as the solvent can hold at a given temperature. Crystallization occurs when a small amount of solute is added. Is a mixture. supersaturated A solution is saturated at 25 g per 100g of solution; this solution has 25 grams in 100 g of solution. It contains more solute molecules than usually can dissolve. Is cloudy. A solution saturated at 25 g per 100 g of solution; this solution has 31 grams in 100 g of solution.
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter8: Solutions
Section: Chapter Questions
Problem 8.7EP: Classify each of the following solutions as saturated, unsaturated, or supersaturated based on the...
Related questions
Question
Can you please answer this and show the reasoning why?
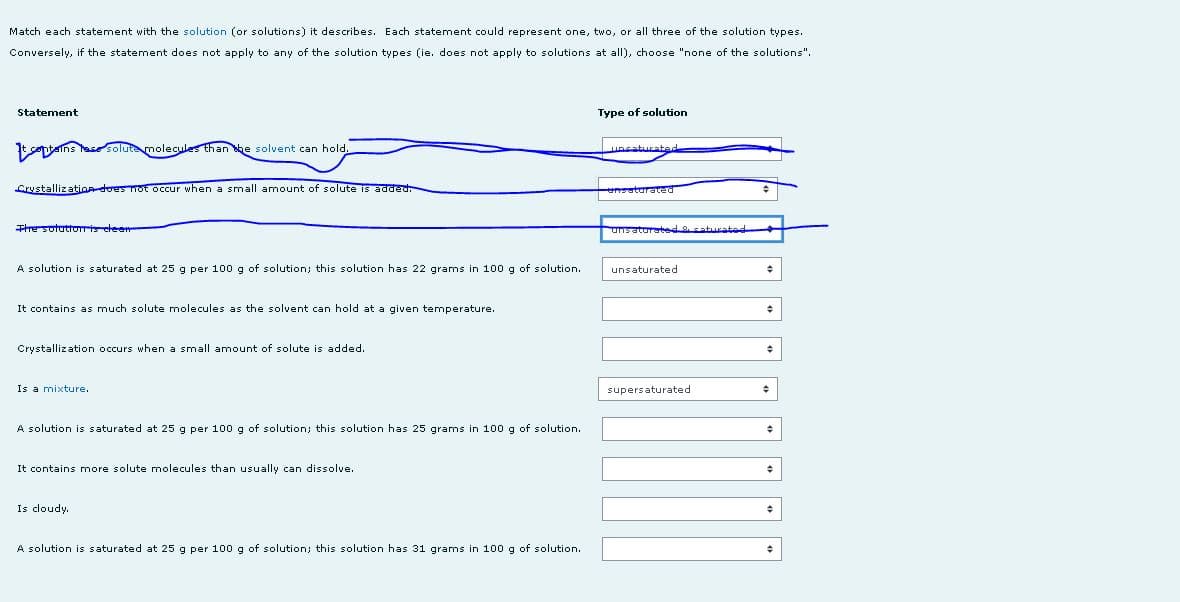
Transcribed Image Text:Match each statement with the solution (or solutions) it describes. Each statement could represent one, two, or all three of the solution types.
Conversely, if the statement does not apply to any of the solution types (ie. does not apply to solutions at all), choose "none of the solutions".
Statement
Type of solution
ains hase solute molecules than he solvent can hold.
uncaturatede
Crystallization-does Tot occur when a small amount of solute is added.
ensaturated
Thesotatto elee
unsaturatedsaturatad
A solution is saturated at 25 g per 100 g of solution; this solution has 22 grams in 100 g of solution.
unsaturated
It contains as much solute molecules as the solvent can hold at a given temperature.
Crystallization occurs when a small amount of solute is added.
Is a mixture.
supersaturated
A solution is saturated at 25 g per 100g of solution; this solution has 25 grams in 100 g of solution.
It contains more solute molecules than usually can dissolve.
Is cloudy.
A solution is saturated at 25 g per 100g of solution; this solution has 31 grams in 100 g of solution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning