Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter16: Reactions Between Acids And Bases
Section: Chapter Questions
Problem 16.7QE
Related questions
Question
Transcribed Image Text:File
Edit
View
History
Bookmarks
Profiles
Tab
Window
Help
...
48%
Thu M
How to make a buffer at a parti X
Aktiv Chemistry
A app.101edu.co
ABP
E Google Docs
AClasses
Google Slides
h Hulu | Home N Netflix
a Amazon.com: Onli...
+ Google Sheets
YouTube
Manage Batches -...
do PollEv
P Academic - inside-
Question 30 of 30
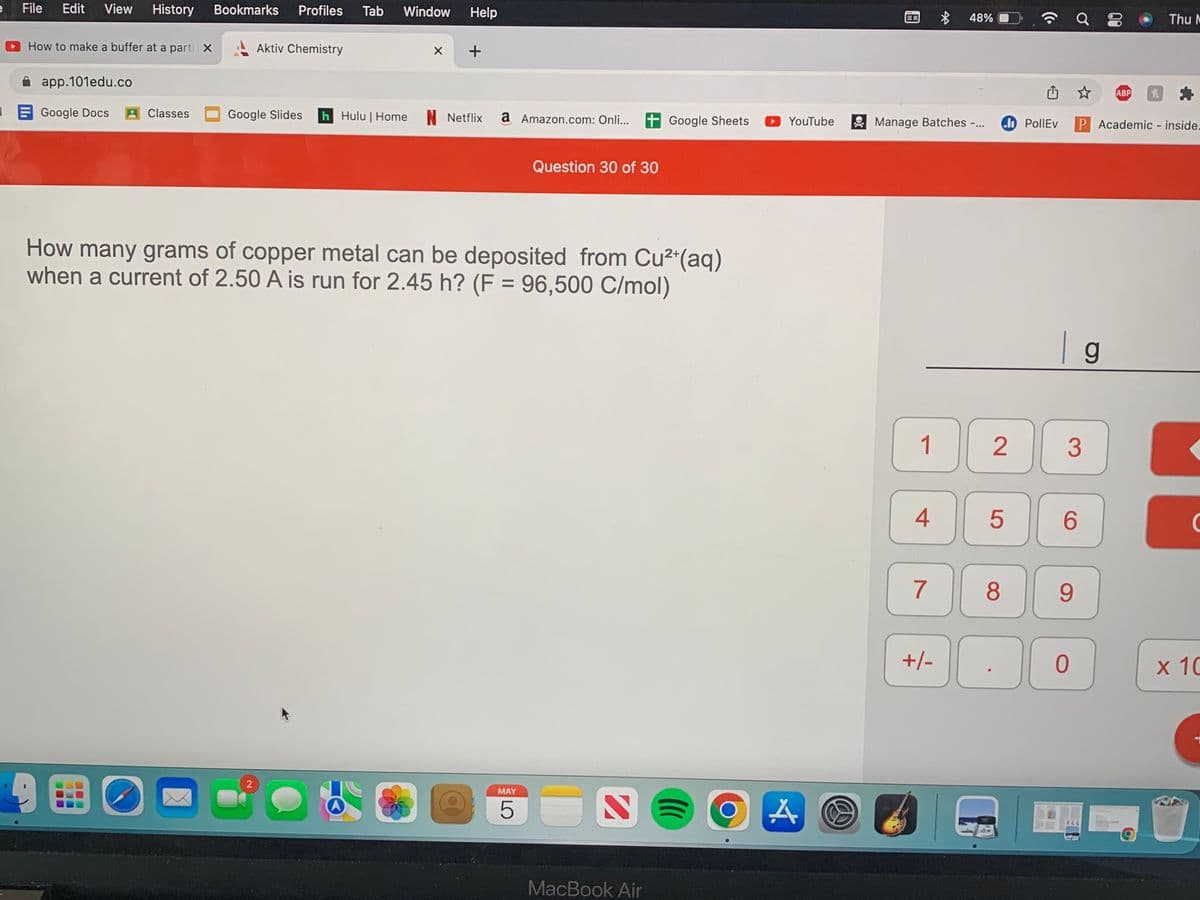
How many grams of copper metal can be deposited from Cu2"(aq)
when a current of 2.50 A is run for 2.45 h? (F = 96,500 C/mol)
| g
1
2
3
4
6
7
8
9.
+/-
х 10
MAY
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning