How many mL of MNSO4 would be produced in the following reaction if you started with 2.00 g H2C2O4, 2.00 g KMNO4 and 2.00 g H2SO4? Density of MNSO4 is 2.66 g/mL. H2C2O4(aq) + KMNO4(aq) + H2SO4(aq) CO2G) + MnS04(aq) + K2SO4(aq) + H2O0)
How many mL of MNSO4 would be produced in the following reaction if you started with 2.00 g H2C2O4, 2.00 g KMNO4 and 2.00 g H2SO4? Density of MNSO4 is 2.66 g/mL. H2C2O4(aq) + KMNO4(aq) + H2SO4(aq) CO2G) + MnS04(aq) + K2SO4(aq) + H2O0)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 90GQ: A Menthol, from oil of mint, has a characteristic odor. The compound contains only C, H, and O. If...
Related questions
Question
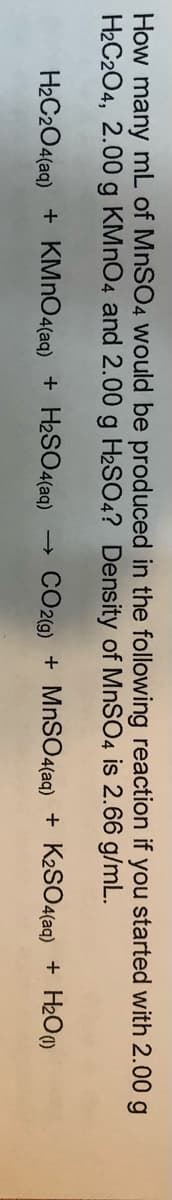
Transcribed Image Text:How many mL of MnSO4 would be produced in the following reaction if you started with 2.00 g
H2C204, 2.00 g KMNO4 and 2.00 g H2SO4? Density of MNSO4 is 2.66 g/mL.
H2C2O4(aq) + KMNO4(aq) + H2SO4(aq)
CO2(g) + MnSO4(aq) + K2SO4(aq) + H2O)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning