How many moles of H2SO, are required to completely react with 7.20 mol of Al according to the balanced chemical reaction: 2 Al(s) + 3 H2SO.(aq) → AL(SO.)3(aq) + 3 H2(g) STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 1 6.022 x 1023 2.02 g H2SO4 7.20 26.98 mol Al2(SO.)= 2 3 mol H2SO, 98.08 3.60 mol H2 342.14 10.8 g Al 21.6 2.40 mol Al
How many moles of H2SO, are required to completely react with 7.20 mol of Al according to the balanced chemical reaction: 2 Al(s) + 3 H2SO.(aq) → AL(SO.)3(aq) + 3 H2(g) STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 1 6.022 x 1023 2.02 g H2SO4 7.20 26.98 mol Al2(SO.)= 2 3 mol H2SO, 98.08 3.60 mol H2 342.14 10.8 g Al 21.6 2.40 mol Al
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 67QAP: When potassium chlorate is subjected to high temperatures, it decomposes into potassium chloride and...
Related questions
Question
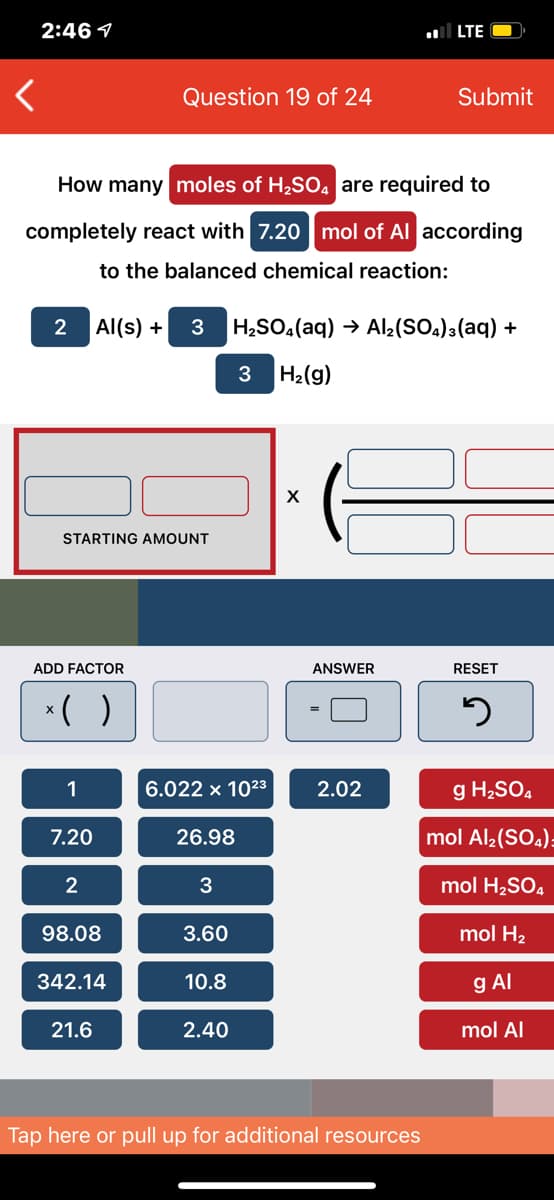
Transcribed Image Text:2:46 1
LTE
Question 19 of 24
Submit
How many moles of H2SO4 are required to
completely react with 7.20 mol of Al according
to the balanced chemical reaction:
2
Al(s) +
3
H¿SO.(aq) → Al2(SOa)3(aq) +
H2(g)
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
1
6.022 x 1023
2.02
g H2SO4
7.20
26.98
mol Al2(SO.)=
2
3
mol H,SO4
98.08
3.60
mol H2
342.14
10.8
g Al
21.6
2.40
mol Al
Tap here or pull up for additional resources
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning