The reaction of ammonia and oxygen is shown below. 4 NH3(aq) + 5 O2(g) 4 NO(g) + 6 H20(1) In an experiment, 2.00 g of ammonia is added to 2.00 g of oxygen in a vessel. The atomic weights (g/mol) are as follows: N = 14.O; H = 1.0; O = 16.0. %3D %3D a. Identify the limiting reactant. For subscripts, use underscore (Ex. water is written as H 20). b. What is the mass in grams of NO? Enter numerical answer only with the correct number of significant figures.
The reaction of ammonia and oxygen is shown below. 4 NH3(aq) + 5 O2(g) 4 NO(g) + 6 H20(1) In an experiment, 2.00 g of ammonia is added to 2.00 g of oxygen in a vessel. The atomic weights (g/mol) are as follows: N = 14.O; H = 1.0; O = 16.0. %3D %3D a. Identify the limiting reactant. For subscripts, use underscore (Ex. water is written as H 20). b. What is the mass in grams of NO? Enter numerical answer only with the correct number of significant figures.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.62PAE: 62 Ammonium dinitramide (ADN), NH4N(NO2)2, was considered as a possible replacement for aluminium...
Related questions
Question
I need help with significant figures in chemistry
Transcribed Image Text:Quizzes > Homework 3
mework 3
ed: Feb 21 at 9:44pm
iz Instructions
5 pts
Question 17
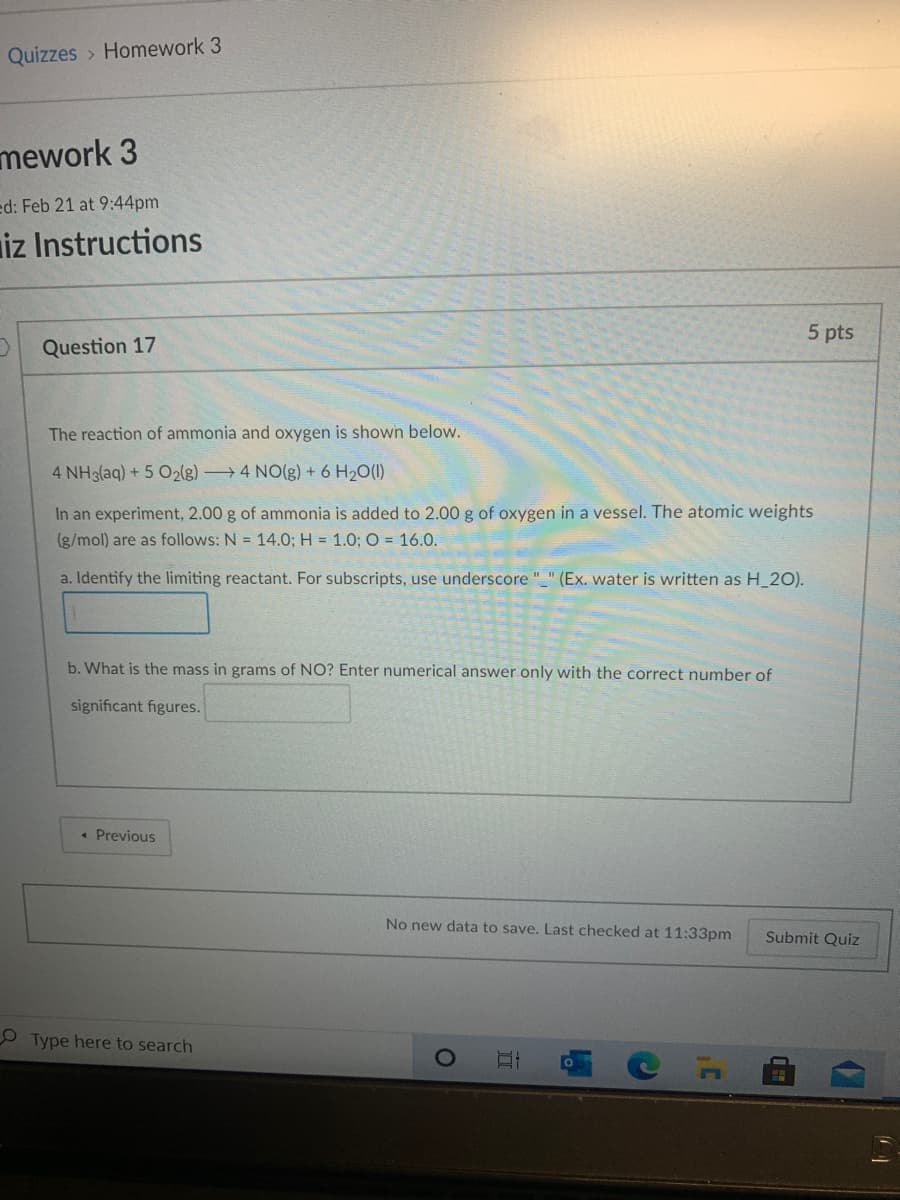
The reaction of ammonia and oxygen is shown below.
4 NH3(aq) + 5 O2(g) 4 NO(g) + 6 H2O(1)
In an experiment, 2.00 g of ammonia is added to 2.00 g of oxygen in a vessel. The atomic weights
(g/mol) are as follows: N = 14.O; H = 1.0; O = 16.0.
a. Identify the limiting reactant. For subscripts, use underscore "_" (Ex. water is written as H_2O).
b. What is the mass in grams of NO? Enter numerical answer only with the correct number of
significant figures.
• Previous
No new data to save. Last checked at 11:33pm
Submit Quiz
O Type here to search
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning