• How many orbitals does each atom have? • Does that match the number of orbitals in its valence shell? • Does the number of orbitals match the number of bonds? • Label the pictures of the orbitals in the table above with the type of bond each can make. • Draw a picture of two sp? carbons forming ethene (CH2CH2) with 4 hydrogen atoms. Draw a picture of a carbon forming methane (CH4) with 4 hydrogen atoms.
• How many orbitals does each atom have? • Does that match the number of orbitals in its valence shell? • Does the number of orbitals match the number of bonds? • Label the pictures of the orbitals in the table above with the type of bond each can make. • Draw a picture of two sp? carbons forming ethene (CH2CH2) with 4 hydrogen atoms. Draw a picture of a carbon forming methane (CH4) with 4 hydrogen atoms.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter3: Electron Orbitals
Section: Chapter Questions
Problem 8E
Related questions
Question
Answer all 7 subparts right below the table
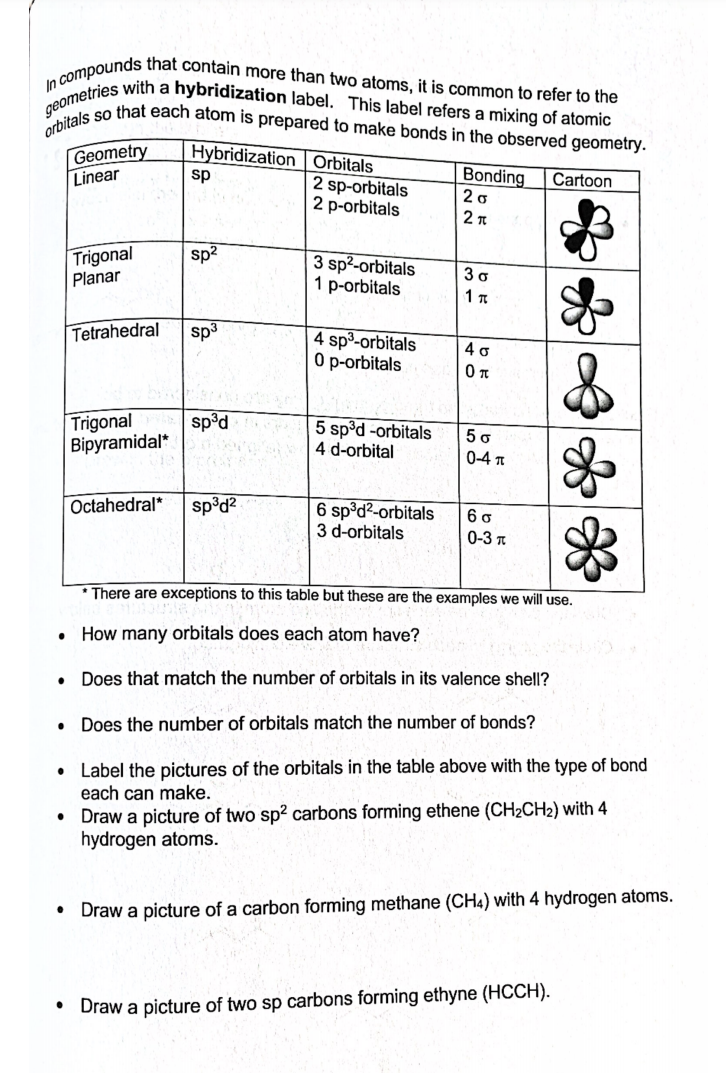
Transcribed Image Text:In compounds that contain more than two atoms, it is common to refer to the
orbitals so that each atom is prepared to make bonds in the observed geometry.
geometries with a hybridization label. This label refers a mixing of atomic
Geometry
Linear
Hybridization Orbitals
Bonding
20
sp
Cartoon
2 sp-orbitals
2 p-orbitals
Trigonal
Planar
sp2
3 sp²-orbitals
1 p-orbitals
3 o
1 T
Tetrahedral
sp3
4 sp-orbitals
0 p-orbitals
4 o
Trigonal
sp°d
5 sp°d -orbitals
4 d-orbital
5 o
Bipyramidal*
0-4 T
Octahedral*
sp³d?
6 sp°d?-orbitals
3 d-orbitals
0-3 T
* There are exceptions to this table but these are the examples we will use.
How many orbitals does each atom have?
• Does that match the number of orbitals in its valence shell?
Does the number of orbitals match the number of bonds?
• Label the pictures of the orbitals in the table above with the type of bond
each can make.
• Draw a picture of two sp? carbons forming ethene (CH2CH2) with 4
hydrogen atoms.
Draw a picture of a carbon forming methane (CH4) with 4 hydrogen atoms.
• Draw a picture of two sp carbons forming ethyne (HCCH).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning