i) ASF3, (ii) BIF3, (iii) C1O3", (iv) BrO2" Answer the following questions for the molecules given above: a) Draw the Lewis structure for each of the following molecules or ions. b) Predict their electron-domain and molecular geometries. c) Assign oxidation numbers and formal charges to each atom. d) Is it polar or nonpolar? e) Comment on the bond angle of each compound. f) Write the hybridization of the central atom in each molecule.
i) ASF3, (ii) BIF3, (iii) C1O3", (iv) BrO2" Answer the following questions for the molecules given above: a) Draw the Lewis structure for each of the following molecules or ions. b) Predict their electron-domain and molecular geometries. c) Assign oxidation numbers and formal charges to each atom. d) Is it polar or nonpolar? e) Comment on the bond angle of each compound. f) Write the hybridization of the central atom in each molecule.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 94AP: The molecular ion S3N3 has the cyclic structure All SN bonds are equivalent. (a) Give six...
Related questions
Question
Can you solve the d, e and f options?
If it includes these in the question, can you solve it using significant figures and dimensional analysis?
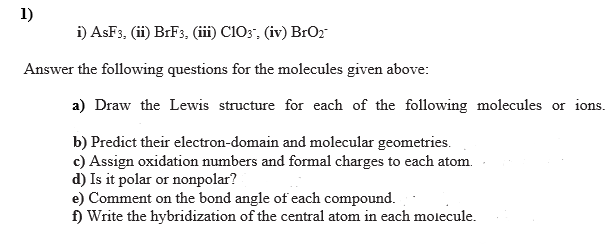
Transcribed Image Text:1)
i) ASF3, (ii) BRF3, (iii) C103", (iv) BrO2
Answer the following questions for the molecules given above:
a) Draw the Lewis structure for each of the following molecules or ions.
b) Predict their electron-domain and molecular geometries.
c) Assign oxidation numbers and formal charges to each atom.
d) Is it polar or nonpolar?
e) Comment on the bond angle of each compound.
f) Write the hybridization of the central atom in each molecule.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning