Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
How much of the excess reactant will be left after the reaction is complete?
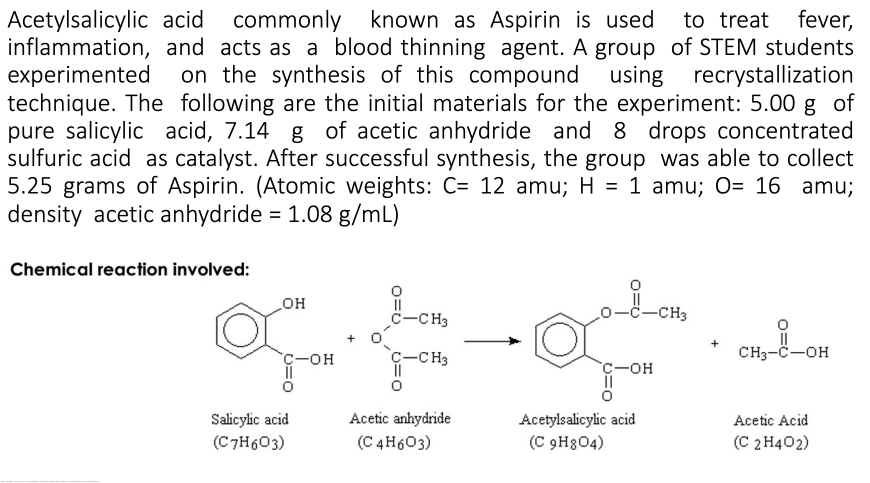
Transcribed Image Text:fever,
Acetylsalicylic acid commonly known as Aspirin is used
inflammation, and acts as a blood thinning agent. A group of STEM students
experimented
technique. The following are the initial materials for the experiment: 5.00 g of
pure salicylic acid, 7.14 g of acetic anhydride and 8 drops concentrated
sulfuric acid as catalyst. After successful synthesis, the group was able to collect
5.25 grams of Aspirin. (Atomic weights: C= 12 amu; H = 1 amu; O= 16 amu;
density acetic anhydride = 1.08 g/mL)
to treat
on the synthesis of this compound
using recrystallization
%3D
Chemical reaction involved:
он
II
c-CH3
ċ–CH3
C-OH
c-CH3
CH3-C-OH
`c-OH
Acetylsalicylic acid
(C 9H8O4)
Acetic anhydride
Salicylic acid
(C7H603)
Acetic Acid
(C 4H603)
(C 2 H402)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole