Hydrogen behaves as an ideal gas at temperatures greater than 200 K and at pressures less than 50 atm. Suppose 6.00 mol hydrogen is initially contained in a 100-L vessel at a pressure of 2.00 atm. The average molar heat capacity of hydrogen at constant pressure, c, is 29.3 J K-' mol-1 in the temperature range of this problem. The gas is cooled reversibly at constant pressure from its initial state to a volume of 50.0 L. Calculate the following quantities for this process. (a) Temperature of the gas in the final state, T2 (b) Work done on the gas, w, in joules (c) Internal energy change of the gas, AU, in joules (d) Heat absorbed by the gas, q, in joules
Hydrogen behaves as an ideal gas at temperatures greater than 200 K and at pressures less than 50 atm. Suppose 6.00 mol hydrogen is initially contained in a 100-L vessel at a pressure of 2.00 atm. The average molar heat capacity of hydrogen at constant pressure, c, is 29.3 J K-' mol-1 in the temperature range of this problem. The gas is cooled reversibly at constant pressure from its initial state to a volume of 50.0 L. Calculate the following quantities for this process. (a) Temperature of the gas in the final state, T2 (b) Work done on the gas, w, in joules (c) Internal energy change of the gas, AU, in joules (d) Heat absorbed by the gas, q, in joules
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
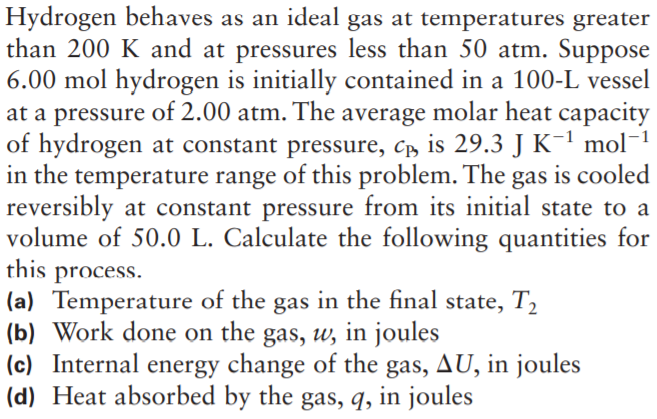
Transcribed Image Text:Hydrogen behaves as an ideal gas at temperatures greater
than 200 K and at pressures less than 50 atm. Suppose
6.00 mol hydrogen is initially contained in a 100-L vessel
at a pressure of 2.00 atm. The average molar heat capacity
of hydrogen at constant pressure, c, is 29.3 J K-' mol-1
in the temperature range of this problem. The gas is cooled
reversibly at constant pressure from its initial state to a
volume of 50.0 L. Calculate the following quantities for
this process.
(a) Temperature of the gas in the final state, T2
(b) Work done on the gas, w, in joules
(c) Internal energy change of the gas, AU, in joules
(d) Heat absorbed by the gas, q, in joules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning