The boiling points of three compounds are tabulated here. Molar Mass Boiling Point 2-hexanone 100.16 128°C heptane 100.20 98°C 1-hexanol 102.17 156°C Answer the following questions without looking up the structures for these molecules: Which compound experiences hydrogen bonding? Which compound is polar but does not experience hydrogen bonding? Which is neither polar nor capable of hydrogen bonding? Explain your answers.
The boiling points of three compounds are tabulated here. Molar Mass Boiling Point 2-hexanone 100.16 128°C heptane 100.20 98°C 1-hexanol 102.17 156°C Answer the following questions without looking up the structures for these molecules: Which compound experiences hydrogen bonding? Which compound is polar but does not experience hydrogen bonding? Which is neither polar nor capable of hydrogen bonding? Explain your answers.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 18CR
Related questions
Question
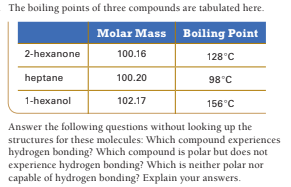
Transcribed Image Text:The boiling points of three compounds are tabulated here.
Molar Mass
Boiling Point
2-hexanone
100.16
128°C
heptane
100.20
98°C
1-hexanol
102.17
156°C
Answer the following questions without looking up the
structures for these molecules: Which compound experiences
hydrogen bonding? Which compound is polar but does not
experience hydrogen bonding? Which is neither polar nor
capable of hydrogen bonding? Explain your answers.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning