the vapo 900- S00 700- 500 400 300 acetic acid – pyrrole - orthoxylene 200 100 100 110 120 temperature, "C Use the graph to answer the following questions: most volatile: choose one Which liquid is the most volatile? Which is the least volatile? least volatile: choose one acetic acid: What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be graded correct. pyrrole: orthaxylene: more Suppose a beaker of pyrrole is put inside a sealed tank containing pyrrole gas at 119. degree Cand 396. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? less the same vapor pressure, torr
the vapo 900- S00 700- 500 400 300 acetic acid – pyrrole - orthoxylene 200 100 100 110 120 temperature, "C Use the graph to answer the following questions: most volatile: choose one Which liquid is the most volatile? Which is the least volatile? least volatile: choose one acetic acid: What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be graded correct. pyrrole: orthaxylene: more Suppose a beaker of pyrrole is put inside a sealed tank containing pyrrole gas at 119. degree Cand 396. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? less the same vapor pressure, torr
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section: Chapter Questions
Problem 33GQ
Related questions
Question
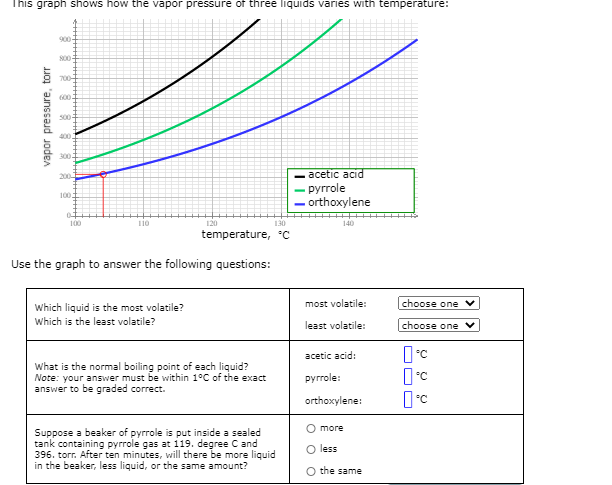
Transcribed Image Text:This graph shows how the vapor pressure of three liquids varies with temperature:
900
S00
700-
600
500-
400.
300
acetic acid
- pyrrole
- orthoxylene
200
100
100
110
120
130
140
temperature, °C
Use the graph to answer the following questions:
most volatile:
choose one v
Which liquid is the most volatile?
Which is the least volatile?
least volatile:
choose one
acetic acid:
What is the normal boiling point of each liquid?
Note: your answer must be within 1°C of the exact
answer to be graded correct.
руrrole:
orthoxylene:
О more
Suppose a beaker of pyrrole is put inside a sealed
tank containing pyrrole gas at 119. degree Cand
396. torr. After ten minutes, will there be more liquid
in the beaker, less liquid, or the same amount?
less
the same
vapor pressure, torr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning