I am allowed to work with someone I just don’t understand this .. Calculations : neatly labeled and organized for each of the following calculations Include correct and significant figures in all calculations Using the Graduated Cylinder Data from Part B. calculate the: 5. Range of the densities of the three samples of saline
I am allowed to work with someone I just don’t understand this .. Calculations : neatly labeled and organized for each of the following calculations Include correct and significant figures in all calculations Using the Graduated Cylinder Data from Part B. calculate the: 5. Range of the densities of the three samples of saline
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
ChapterA: Scientific Notation And Experimental Error
Section: Chapter Questions
Problem 9P
Related questions
Question
I am allowed to work with someone I just don’t understand this ..
Calculations : neatly labeled and organized for each of the following calculations Include correct and significant figures in all calculations Using the Graduated Cylinder Data from Part B. calculate the:
5. Range of the densities of the three samples of saline
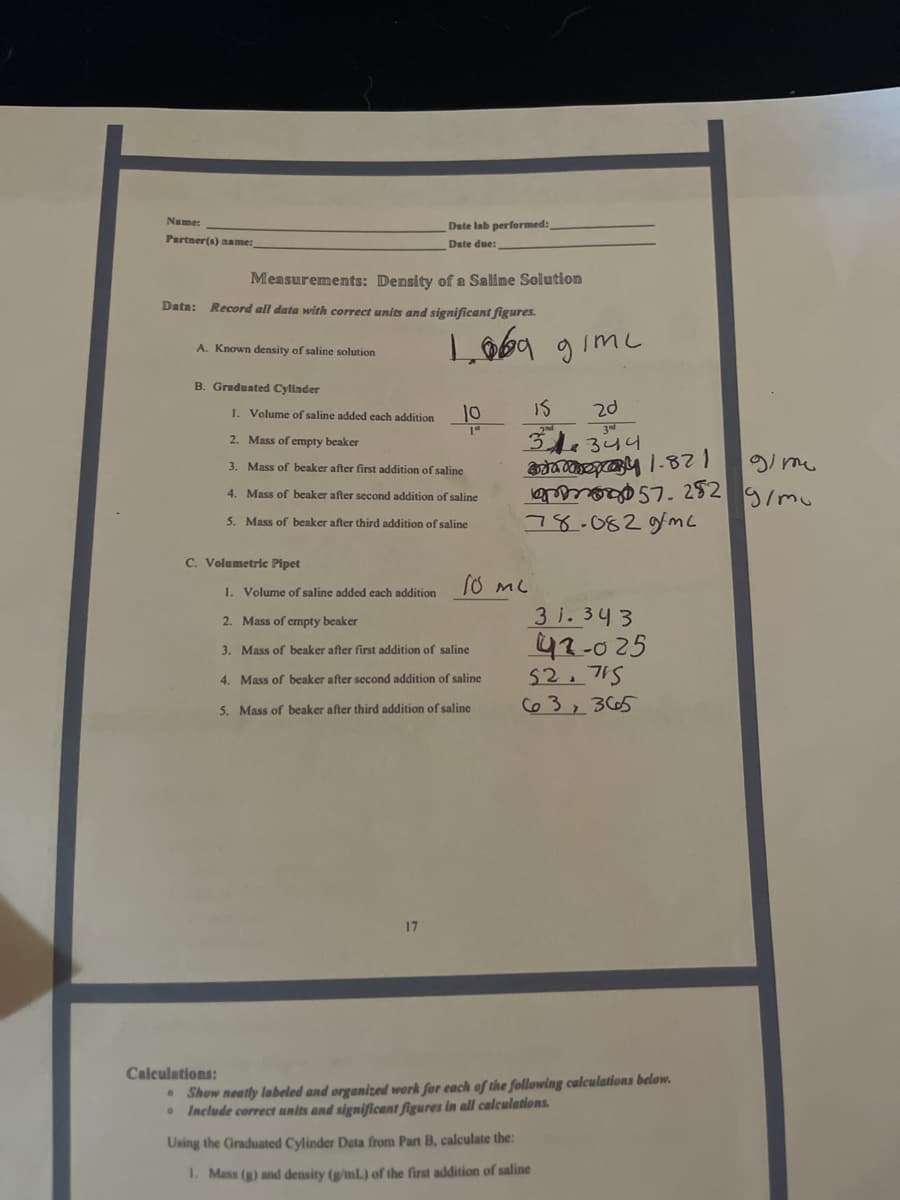
Transcribed Image Text:Name:
Date lab performed:
Partner(s) name:
Date due:
Measurements: Density of a Saline Solution
Data:
Record all data with correct units and significant figures.
Loba gimc
A. Known density of saline solution
B. Graduated Cylinder
1. Volume of saline added each addition
10
IS
20
2. Mass of empty beaker
gi me
3. Mass of beaker after first addition of saline
. 252
78.082gmc
4. Mass of beaker after second addition of saline
5. Mass of beaker after third addition of saline
C. Volumetric Pipet
1. Volume of saline added each addition
3 1. 343
42-025
52.715
Co 3, 365
2. Mass of empty beaker
3. Mass of beaker after first addition of saline
4. Mass of beaker after second addition of saline
5. Mass of beaker after third addition of saline
17
Calculations:
• Show neatly labeled and organized work for each of the following calculations below.
• Include correct units and signmificant figures in all calculations.
Using the Giraduated Cylinder Data from Part B, calculate the:
1. Mass (g) and density (g/mL) of the first addition of saline
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning