I arther: Chair conformers Monosubstituted rings The A-value for methyl, ethyl, isopropyl, and t-butyl substitution on a cyclohexane ring are 7.1kJ/mol, 7.3 kJ/mol, 9.0kJ/mol, and 18.8 kJ/mol, respectively.. Me Me 1. Et 95:5 95:5 Bu Bu 97:3 9995:5 Build the molecules and use your model to explain why the increased size of the substituent doe- not have a dramatic effect until you reach t-butyl. In other words, is it possible for the first three substituents (methyl, ethyl, and isopropyl) to achieve conformation in which 1,3-diaxial interactions are minimized? has -value: 0 9kJ/mol. Build the molecule in order to deterr
I arther: Chair conformers Monosubstituted rings The A-value for methyl, ethyl, isopropyl, and t-butyl substitution on a cyclohexane ring are 7.1kJ/mol, 7.3 kJ/mol, 9.0kJ/mol, and 18.8 kJ/mol, respectively.. Me Me 1. Et 95:5 95:5 Bu Bu 97:3 9995:5 Build the molecules and use your model to explain why the increased size of the substituent doe- not have a dramatic effect until you reach t-butyl. In other words, is it possible for the first three substituents (methyl, ethyl, and isopropyl) to achieve conformation in which 1,3-diaxial interactions are minimized? has -value: 0 9kJ/mol. Build the molecule in order to deterr
Chapter4: Organic Compounds: Cycloalkanes And Their Stereochemistry
Section4.SE: Something Extra
Problem 24VC: A trisubstituted cyclohexane with three substituents-red, green, and blue-undergoes a ring-flip to...
Related questions
Question
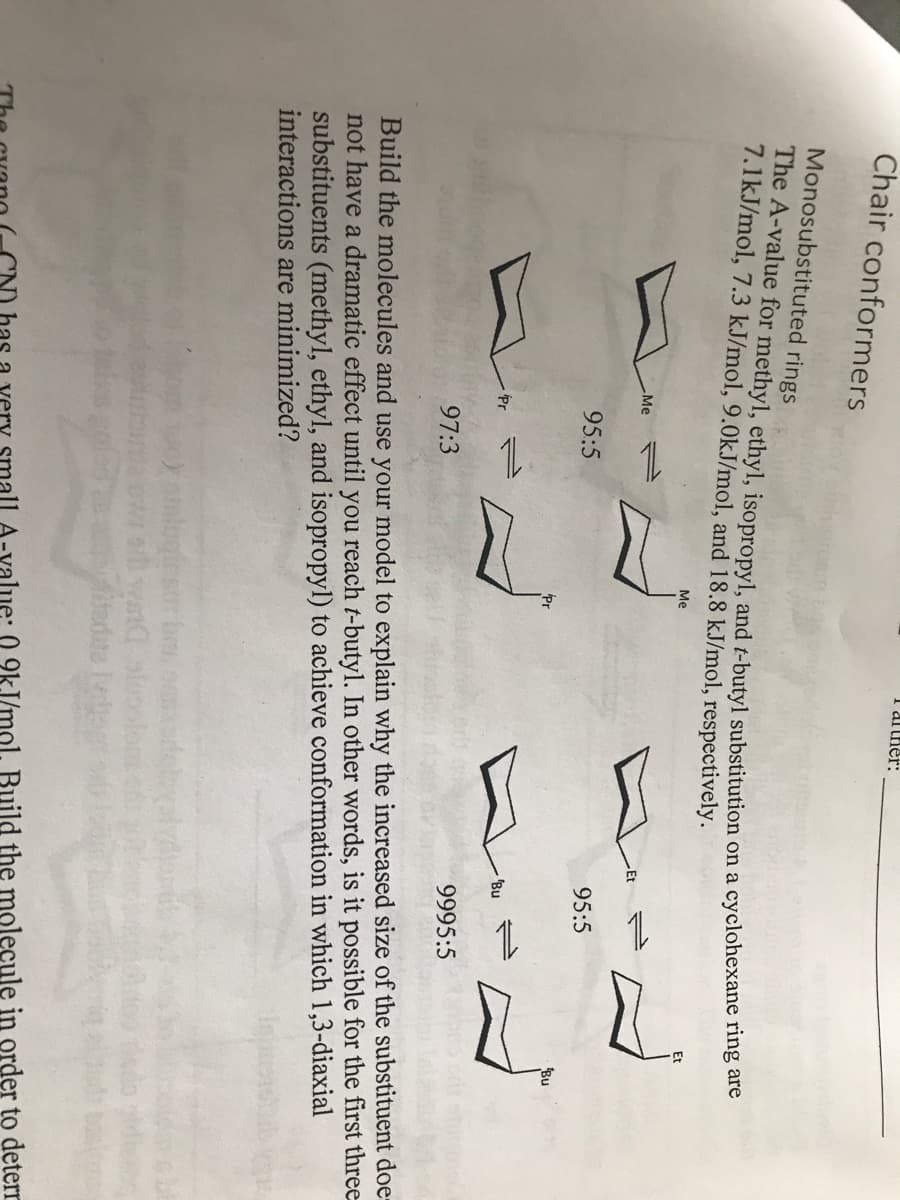
Transcribed Image Text:I arther:
Chair conformers
Monosubstituted rings
The A-value for methyl, ethyl, isopropyl, and t-butyl substitution on a cyclohexane ring are
7.1kJ/mol, 7.3 kJ/mol, 9.0kJ/mol, and 18.8 kJ/mol, respectively..
Me
Me
1.
Et
95:5
95:5
Bu
Bu
97:3
9995:5
Build the molecules and use your model to explain why the increased size of the substituent doe-
not have a dramatic effect until you reach t-butyl. In other words, is it possible for the first three
substituents (methyl, ethyl, and isopropyl) to achieve conformation in which 1,3-diaxial
interactions are minimized?
mlugiest bn
todte
has
-value: 0 9k.J/mol. Build the molecule in order to deterr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning