i bartleby.com/questions-and-answers/1-potassium-phosphate-with-copper-ii-acetate-2-sodium-sulfide-with-iron-i-nitrate-3-cesium-hydroxi/0583850f-6991-4155-ae7e-8cafe9a09bfc A Maps > YouTube Pakistani Women Br. = bartleby Q Search for textbooks, step-by-step explanations to homework questions,.. E Ask an Expert 1) potassium phosphate with copper (II) acetate - + Q&A Library 2) sodium sulfide with iron (I) nitrate 4) nickel (III) chloride and lead (I) nitra 3) cesium hydroxide and chromic acid Ask an Expert 5) chromium (I) bromide with lithium o 4) nickel () chloride and lead (II) nitrate. 30 of 30 questions left this cycle Renews on Oct 19, 2021 6) sulfuric acid with rubidium hydroxide sa chromium (II) bromide with lithium oxalate a esponse times may vary by subject and question plexity. Median response time is 34 minutes for paid scribers and may be longer for promotional offers. 7) barium hydroxide with perchloric acid 6) sulfuric acid with rubidium hydroxide 8) cesium chromate with rubidium oxide 7) barium hydroxide with perchloric acid- 9) sodium fluoride with nickel (I) sulfa in 8) cesium chromate with rubidium oxide. Mcarbon
i bartleby.com/questions-and-answers/1-potassium-phosphate-with-copper-ii-acetate-2-sodium-sulfide-with-iron-i-nitrate-3-cesium-hydroxi/0583850f-6991-4155-ae7e-8cafe9a09bfc A Maps > YouTube Pakistani Women Br. = bartleby Q Search for textbooks, step-by-step explanations to homework questions,.. E Ask an Expert 1) potassium phosphate with copper (II) acetate - + Q&A Library 2) sodium sulfide with iron (I) nitrate 4) nickel (III) chloride and lead (I) nitra 3) cesium hydroxide and chromic acid Ask an Expert 5) chromium (I) bromide with lithium o 4) nickel () chloride and lead (II) nitrate. 30 of 30 questions left this cycle Renews on Oct 19, 2021 6) sulfuric acid with rubidium hydroxide sa chromium (II) bromide with lithium oxalate a esponse times may vary by subject and question plexity. Median response time is 34 minutes for paid scribers and may be longer for promotional offers. 7) barium hydroxide with perchloric acid 6) sulfuric acid with rubidium hydroxide 8) cesium chromate with rubidium oxide 7) barium hydroxide with perchloric acid- 9) sodium fluoride with nickel (I) sulfa in 8) cesium chromate with rubidium oxide. Mcarbon
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
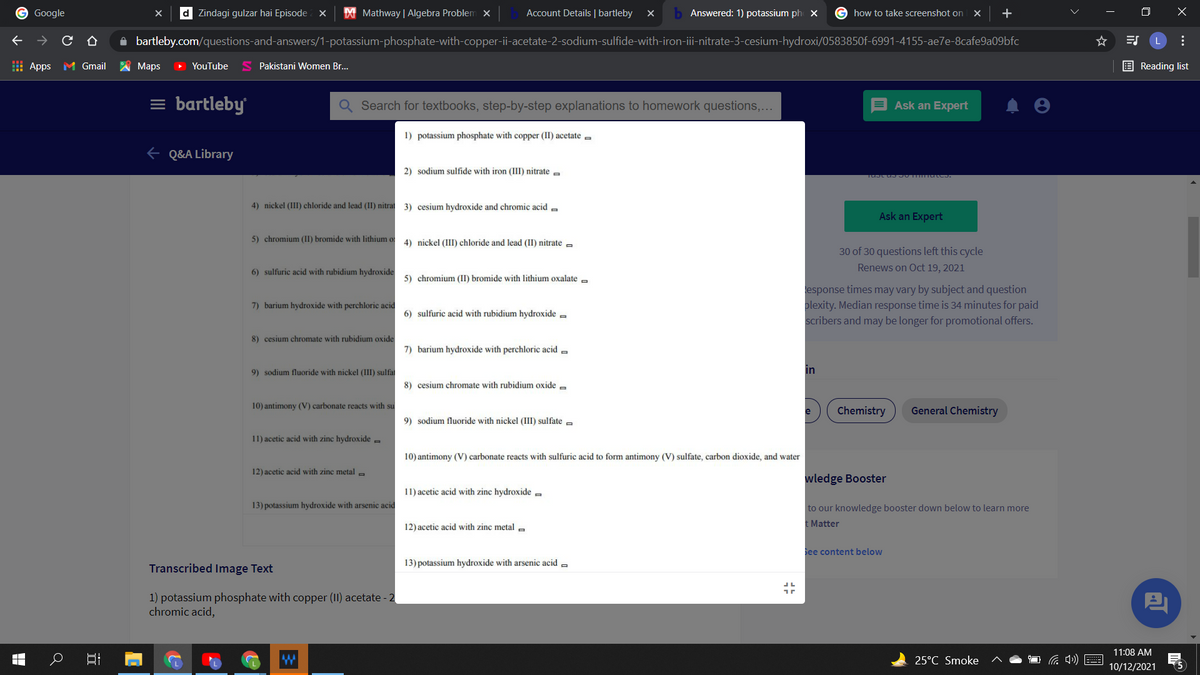
write the molecular ,ionic and net ionic equation for followings
Transcribed Image Text:G Google
d Zindagi gulzar hai Episode
X Mathway | Algebra Problem x
Account Details | bartleby
b Answered: 1) potassium ph ×
G how to take screenshot on
i bartleby.com/questions-and-answers/1-potassium-phosphate-with-copper-ii-acetate-2-sodium-sulfide-with-iron-ii-nitrate-3-cesium-hydroxi/0583850f-6991-4155-ae7e-8cafe9a09bfc
L
I Apps
M Gmail
A Maps
YouTube
Pakistani Women Br..
E Reading list
= bartleby
Search for textbooks, step-by-step explanations to homework questions,...
Ask an Expert
1) potassium phosphate with copper (II) acetate -
e Q&A Library
2) sodium sulfide with iron (III) nitrate a
4) nickel (III) chloride and lead (II) nitrat 3) cesium hydroxide and chromic acid a
Ask an Expert
5) chromium (II) bromide with lithium o 4) nickel (III) chloride and lead (II) nitrate a
30 of 30 questions left this cycle
Renews on Oct 19, 2021
6) sulfuric acid with rubidium hydroxide
5) chromium (II) bromide with lithium oxalate
esponse times may vary by subject and question
plexity. Median response time is 34 minutes for paid
scribers and may be longer for promotional offers.
7) barium hydroxide with perchloric acid
6) sulfuric acid with rubidium hydroxide
8) cesium chromate with rubidium oxide
7) barium hydroxide with perchloric acid =
9) sodium fluoride with nickel (III) sulfa
in
8) cesium chromate with rubidium oxide -
10) antimony (V) carbonate reacts with su
le
Chemistry
General Chemistry
9) sodium fluoride with nickel (III) sulfate a
11) acetic acid with zinc hydroxide -
10) antimony (V) carbonate reacts with sulfuric acid to form antimony (V) sulfate, carbon dioxide, and water
12) acetic acid with zine metal a
wledge Booster
11) acetic acid with zinc hydroxide =
13) potassium hydroxide with arsenic acid
to our knowledge booster down below to learn more
t Matter
12) acetic acid with zinc metal a
See content below
13) potassium hydroxide with arsenic acid -
Transcribed Image Text
1) potassium phosphate with copper (II) acetate - 2
chromic acid,
四
11:08 AM
25°C Smoke
5
10/12/2021
...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY