Safari File Edit View History Bookmarks Window Help 4)) 51% O Sun 10:43 AM session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H...
Safari File Edit View History Bookmarks Window Help 4)) 51% O Sun 10:43 AM session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H...
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
Transcribed Image Text:Safari
File
Edit
View
History Bookmarks Window Help
4)) 51% O
Sun 10:43 AM
session.masteringchemistry.com
Consider summer class...
Inbox (13) - thesym1@g...
Class Schedule Listing
Schedule 111.009 & 111...
Ellucian Degree Works...
Pearson's MyLab & Mas...
ALEKS - Sofia Simmons...
MasteringChemistry: H...
<Homework 5
+ Molarity
1 of 24
<.
I Review I Constants I Periodic Table
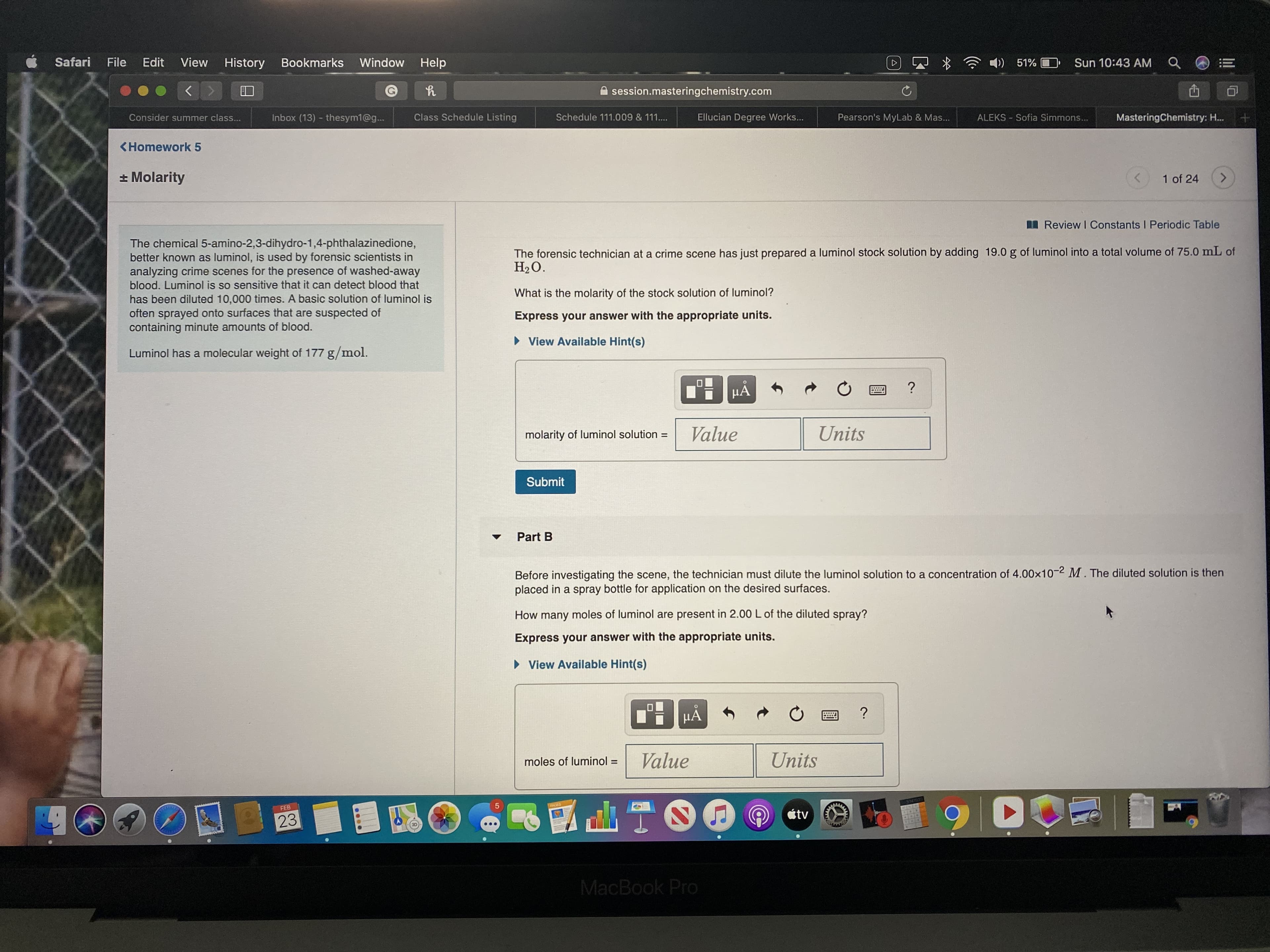
The chemical 5-amino-2,3-dihydro-1,4-phthalazinedione,
better known as luminol, is used by forensic scientists in
analyzing crime scenes for the presence of washed-away
blood. Luminol is so sensitive that it can detect blood that
The forensic technician at a crime scene has just prepared a luminol stock solution by adding 19.0 g of luminol into a total volume of 75.0 mL of
H2O.
What is the molarity of the stock solution of luminol?
has been diluted 10,000 times. A basic solution of luminol is
often sprayed onto surfaces that are suspected of
containing minute amounts of blood.
Express your answer with the appropriate units.
• View Available Hint(s)
Luminol has a molecular weight of 177 g/mol.
HA
molarity of luminol solution
Value
Units
Submit
Part B
Before investigating the scene, the technician must dilute the luminol solution to a concentration of 4.00x10-2 M . The diluted solution is then
placed in a spray bottle for application on the desired surfaces.
How many moles of luminol are present in 2.00 L of the diluted spray?
Express your answer with the appropriate units.
• View Available Hint(s)
HA
moles of luminol :
Value
Units
EGO
23
PAGES
FEB
étv
MacBook Pro
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning