i) In Table 1 (column 3) determine the number of 1H NMR peaks (ignore splitting) that you would expect for each of the above (hint: the no. of different proton environments) and ii) (column 4) predict the simple splitting pattern for each type of proton.
i) In Table 1 (column 3) determine the number of 1H NMR peaks (ignore splitting) that you would expect for each of the above (hint: the no. of different proton environments) and ii) (column 4) predict the simple splitting pattern for each type of proton.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter16: An Introduction To Infrared Spectrometry
Section: Chapter Questions
Problem 16.12QAP
Related questions
Question
100%
Q2. i) In Table 1 (column 3) determine the number of 1H NMR peaks (ignore splitting) that you would expect for each of the above (hint: the no. of different proton environments) and ii) (column 4) predict the simple splitting pattern for each type of proton.
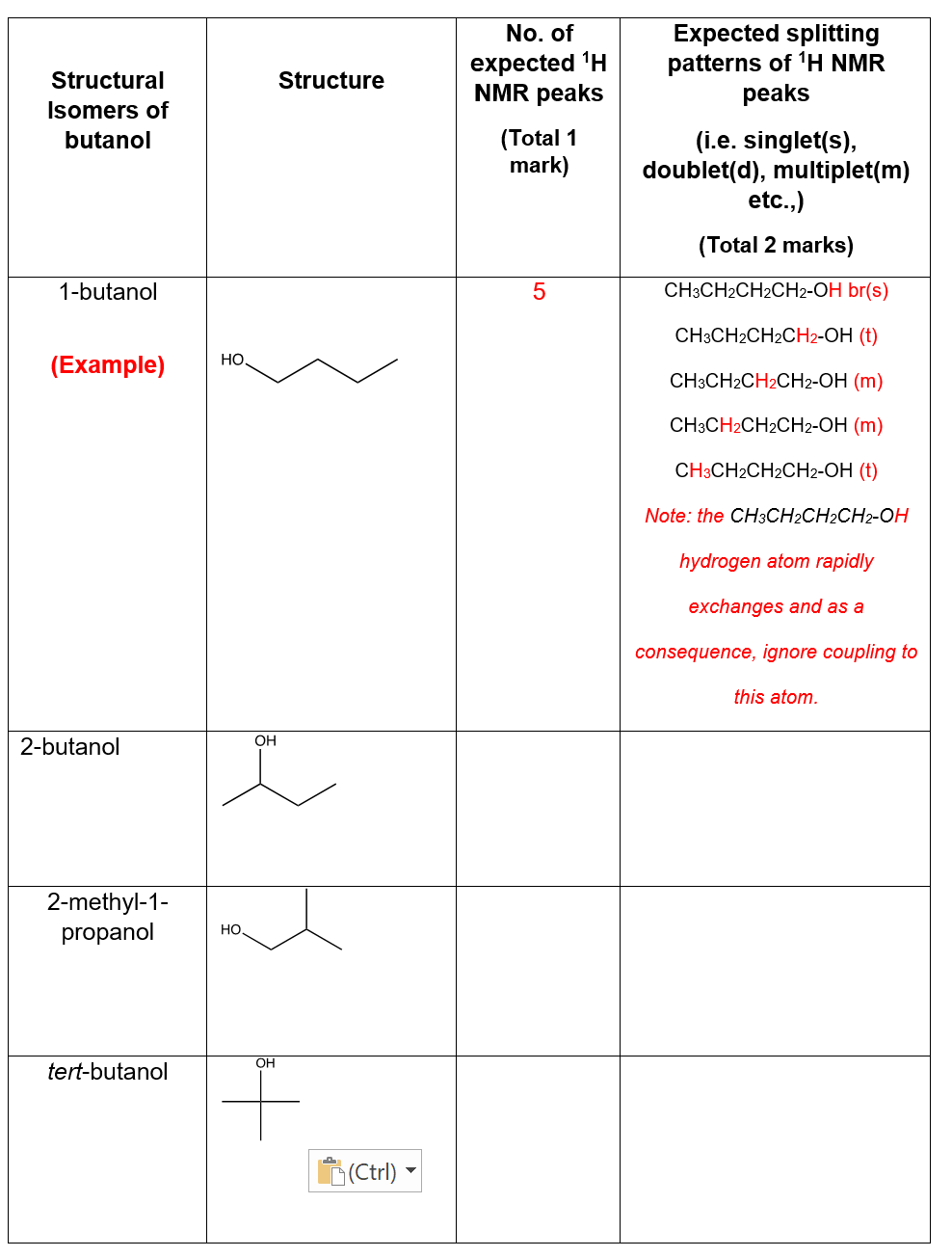
Transcribed Image Text:Structural
Isomers of
butanol
1-butanol
(Example)
2-butanol
2-methyl-1-
propanol
tert-butanol
HO
HO
OH
OH
Structure
(Ctrl) -
No. of
expected ¹H
NMR peaks
(Total 1
mark)
5
Expected splitting
patterns of ¹H NMR
peaks
(i.e. singlet(s),
doublet(d), multiplet(m)
etc.,)
(Total 2 marks)
CH3CH2CH2CH2-OH br(s)
CH3CH2CH2CH2-OH (t)
CH3CH2CH2CH2-OH (m)
CH3CH2CH2CH2-OH (m)
CH3CH2CH2CH2-OH (t)
Note: the CH3CH2CH2CH2-OH
hydrogen atom rapidly
exchanges and as a
consequence, ignore coupling to
this atom.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning