I Review I Constants I Periodic Table Standard Enthalpy of Formation at 289 K Substance Formula AH; (kJ/mol) Part A Hydrogen HBr(g) -36.26 Hypochlorous acid (HOCI) can decompose in the gas phase according to the following balanced equation: bromide Hydrogen chloride HCI(g) -92.30 2 HOCI(g) → 2 HCI(g) + O2(g) AH = -31.0 kJ (T = 298 K, P = 1 atm) Hydrogen fluoride HF(g) -268.60 Using the enthalpy of this reaction and data in the following table, calculate the standard enthalpy of formation for HOCI(g). Hydrogen iodide HI(g) 25.9 Methane CH4(g) -74.80 O -123 kJ/mol +61.3 kJ/mol Water vapor H20(g) -241.8 -76.8 kJ/mol -154 kJ/mol O +76.8 kJ/mol Submit Request Answer
I Review I Constants I Periodic Table Standard Enthalpy of Formation at 289 K Substance Formula AH; (kJ/mol) Part A Hydrogen HBr(g) -36.26 Hypochlorous acid (HOCI) can decompose in the gas phase according to the following balanced equation: bromide Hydrogen chloride HCI(g) -92.30 2 HOCI(g) → 2 HCI(g) + O2(g) AH = -31.0 kJ (T = 298 K, P = 1 atm) Hydrogen fluoride HF(g) -268.60 Using the enthalpy of this reaction and data in the following table, calculate the standard enthalpy of formation for HOCI(g). Hydrogen iodide HI(g) 25.9 Methane CH4(g) -74.80 O -123 kJ/mol +61.3 kJ/mol Water vapor H20(g) -241.8 -76.8 kJ/mol -154 kJ/mol O +76.8 kJ/mol Submit Request Answer
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 104AE
Related questions
Question
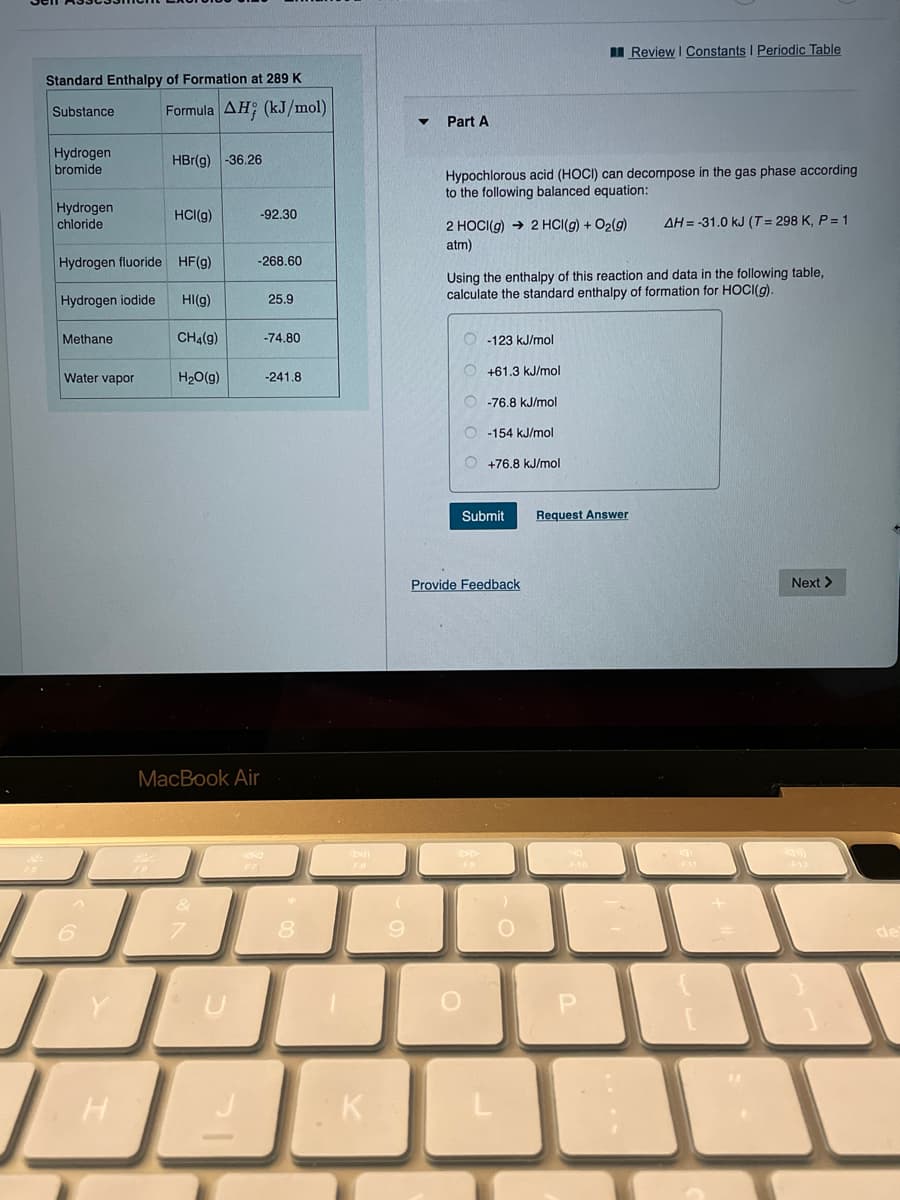
Transcribed Image Text:I Review I Constants I Periodic Table
Standard Enthalpy of Formation at 289 K
Substance
Formula AH; (kJ/mol)
Part A
Hydrogen
bromide
HBr(g) -36.26
Hypochlorous acid (HOCI) can decompose in the gas phase according
to the following balanced equation:
Hydrogen
chloride
HCI(g)
-92.30
2 HOCI(g) → 2 HCI(g) + O2(g)
AH = -31.0 kJ (T = 298 K, P = 1
atm)
Hydrogen fluoride HF(g)
-268.60
Using the enthalpy of this reaction and data in the following table,
calculate the standard enthalpy of formation for HOCI(g).
Hydrogen iodide
HI(g)
25.9
Methane
CH4(g)
-74.80
O -123 kJ/mol
+61.3 kJ/mol
Water vapor
H20(g)
-241.8
O -76.8 kJ/mol
-154 kJ/mol
+76.8 kJ/m
Submit
Request Answer
Provide Feedback
Next >
MacBook Air
K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning