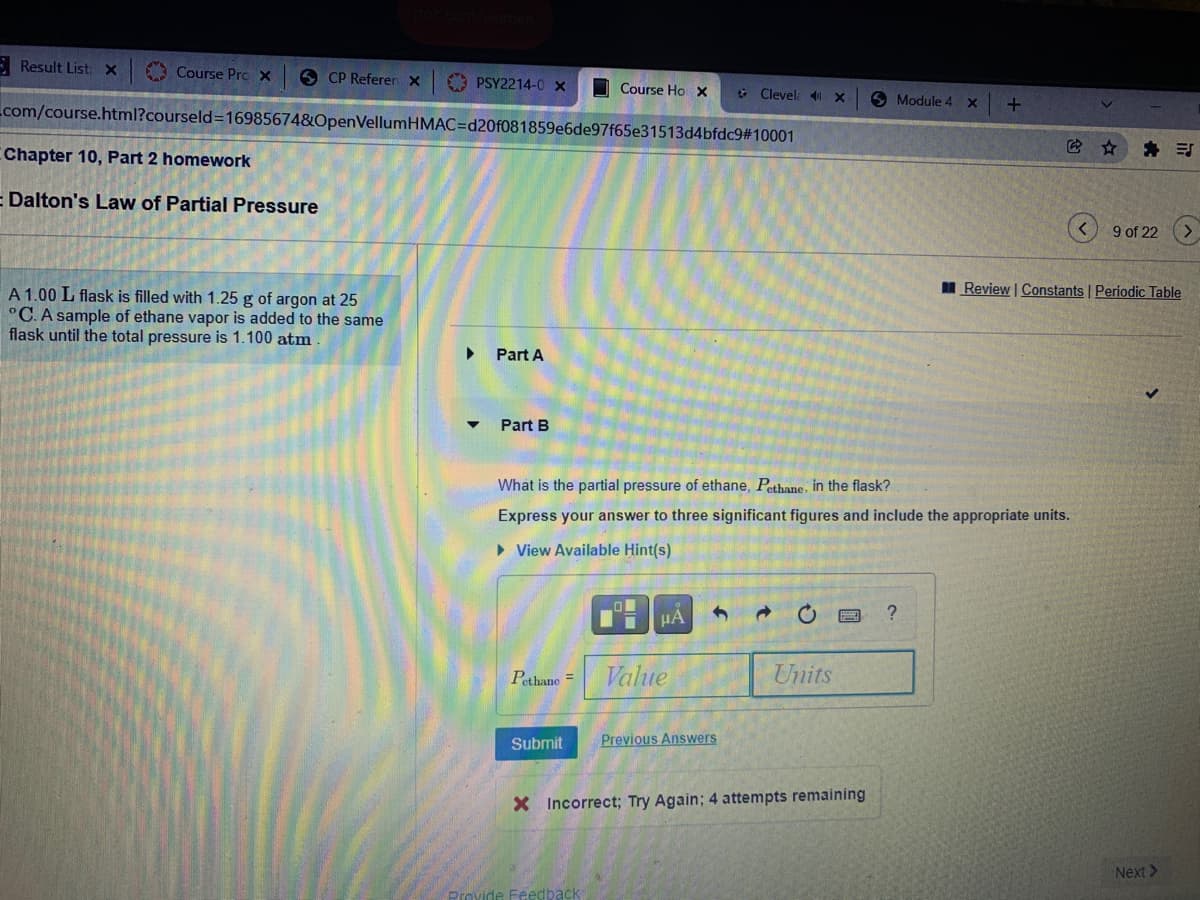
I Review | Constant A 1.00 L flask is filled with 1.25 g of argon at 25 °C. A sample of ethane vapor is added to the same flask until the total pressure is 1.100 atm Part A Part B What is the partial pressure of ethane, Pethane, in the flask? Express your answer to three significant figures and include the appropriate units.
I Review | Constant A 1.00 L flask is filled with 1.25 g of argon at 25 °C. A sample of ethane vapor is added to the same flask until the total pressure is 1.100 atm Part A Part B What is the partial pressure of ethane, Pethane, in the flask? Express your answer to three significant figures and include the appropriate units.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.96PAE
Related questions
Question
Transcribed Image Text:E Result List: x
O Course Prc X
6 CP Referen X
O PSY2214-0 X
O Course Ho x
* Clevel
O Module 4
Lcom/course.html?courseld=16985674&OpenVellumHMAC=d20f081859e6de97f65e31513d4bfdc9#10001
Chapter 10, Part 2 homework
E Dalton's Law of Partial Pressure
9 of 22
I Review | Constants | Periodic Table
A 1.00 L flask is filled with 1.25 g of argon at 25
°C. A sample of ethane vapor is added to the same
flask until the total pressure is 1.100 atm
Part A
Part B
What is the partial pressure of ethane, Pethane, in the flask?
Express your answer to three significant figures and include the appropriate units.
> View Available Hint(s)
HA
?
Pethane =
Value
Units
Submit
Previous Answers
X Incorrect; Try Again; 4 attempts remaining
Next>
Piovide Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning