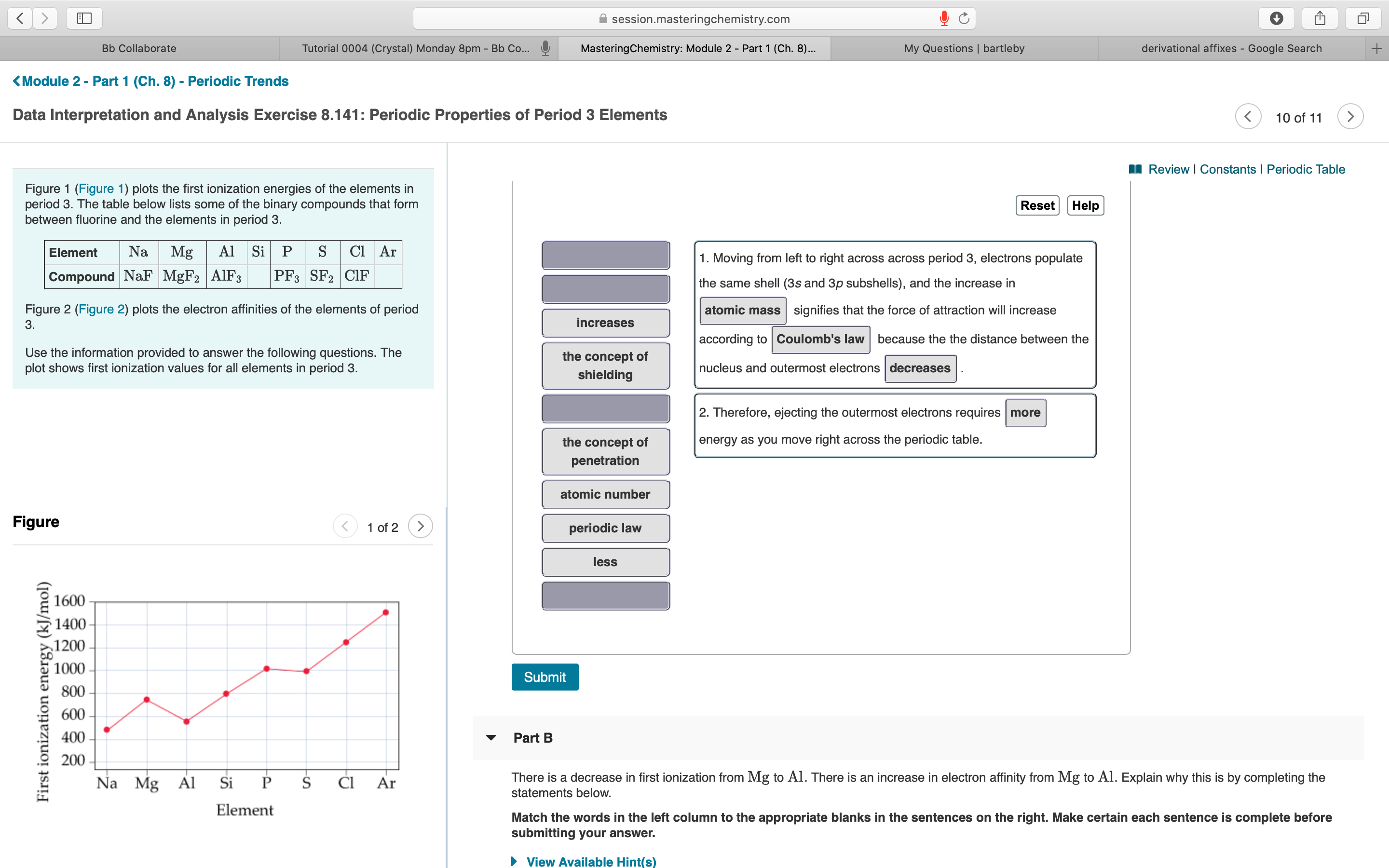
I Review | Constants I Periodic Table Figure 1 (Figure 1) plots the first ionization energies of the elements in period 3. The table below lists some of the binary compounds that form between fluorine and the elements in period 3. Reset Help Element Na Mg Al Si P S Cl Ar 1. Moving from left to right across across period 3, electrons populate Compound NaF MGF2 AIF3 PF3 SF2 ClIF the same shell (3s and 3p subshells), and the increase in atomic mass signifies that the force of attraction will increase Figure 2 (Figure 2) plots the electron affinities of the elements of period 3. increases according to Coulomb's law because the the distance between the Use the information provided to answer the following questions. The plot shows first ionization values for all elements in period 3. the concept of nucleus and outermost electrons decreases shielding 2. Therefore, ejecting the outermost electrons requires more the concept of energy as you move right across the periodic table. penetration atomic number Figure 1 of 2 1 periodic law less 1600 1400 - 1200 1000 - Submit 800 600- 400 Part B 200 Na Mg Al Si P ČI Ar There is a decrease in first ionization from Mg to Al. There is an increase in electron affinity from Mg to Al. Explain why this is by completing the statements below. Element Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. First ionization energy (kJ/mol)
Electron Affinity
When an element undergoes a chemical reaction, it either gains energy or loses energy. This gain or loss of energy is due to the phenomena that occur at atomic level. During reaction, atoms either gain electrons from other atoms or lose electrons to other atoms, and in that process, energy is produced.
P-Block Elements
Elements which are present on the right side of the periodic table are called p-block elements. In addition to the noble gases, they include the families of boron, mercury, nitrogen, oxygen and fluorine. These elements have diverse real-life implementations that we regularly experience around us.
Metals and Non-metals
The periodic table is composed of metals, semi-metals and nonmetal elements. The physical and chemical properties of metals and nonmetals differ from each other. The study of metals and nonmetals will help one to understand the appropriate application of the particular element.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









