Part I Atomic Struwcture I. Draw four protons in the atom to the right. Label them with thecir charge. Insert - Text boxes to "draw" and label 2. Draw five neutrons in the atom to the right. Label them with their charge. Insert- Text boxes to "draw" and label 3. Draw four clectrons in the atom to the right. Place two on the first energy leyel and twogn the socond. Lahel them with their charpe. Insert- Text boxes to "draw and label 4. What clement did you just draw? Add Text Here 5. How do you know? Add Text Here
Part I Atomic Struwcture I. Draw four protons in the atom to the right. Label them with thecir charge. Insert - Text boxes to "draw" and label 2. Draw five neutrons in the atom to the right. Label them with their charge. Insert- Text boxes to "draw" and label 3. Draw four clectrons in the atom to the right. Place two on the first energy leyel and twogn the socond. Lahel them with their charpe. Insert- Text boxes to "draw and label 4. What clement did you just draw? Add Text Here 5. How do you know? Add Text Here
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter3: Chemical Foundations: Elements, Atoms, And Ions
Section: Chapter Questions
Problem 17A
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
This is not graded! Do numbers 1, 2, 3, 4 and 5.
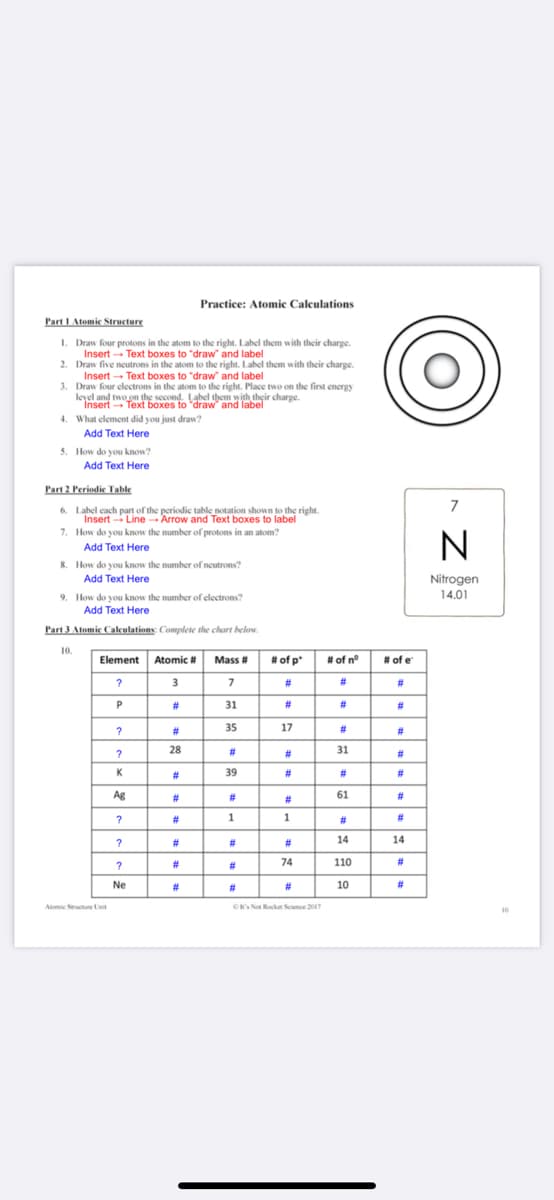
Transcribed Image Text:Practice: Atomic Calculations
Part I Atomic Structure
I. Draw four protons in the atom to the right. Label them with their charge.
Insert - Text boxes to "draw and label
2. Draw five neutrons in the atom to the right. Label them with their charge.
Insert - Text boxes to "draw" and label
3. Draw four electrons in the atom to the right. Place two on the first energy
leyel and two on the second. Label them with their charge.
Insert Text boxes to "draw' and label
4. What element did you just draw?
Add Text Here
5. How do you know?
Add Text Here
Part 2 Periodie Table
7
6. Label cach part of the periodic table notation shown to the right.
Insert - Line Arrow and Text boxes to label
7. How do you know the number of protons in an atom?
Add Text Here
8. How do you know the number of neutrons?
Add Text Here
Nitrogen
14.01
9. How do you know the number of electrons?
Add Text Here
Part 3 Atomic Calculations: Complete the chart below.
10.
Element Atomic # Mass #
# of p"
# of n°
# of e
3
#3
#3
#3
23
31
#
#3
#3
#
35
17
#3
#
%23
28
%23
#
31
#3
K
#3
39
#3
#3
#3
Ag
23
#3
61
#3
#3
#
1
1
#3
#
#3
%23
#3
14
14
#3
%23
74
110
#3
Ne
23
%23
#
10
#
C's No Rocke Seence 2017
Atomic Seructure Lt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
Expert Answers to Latest Homework Questions
Q: You are conducting a small IT project. The duration of this project is 30 days, and you have…
Q: With the aid of diagrams, show and explain how phase locking is used to synchronise an undersampled…
Q: Explain four different methods to develop a work breakdown structure?
Q: A bandwidth-limited radio signal has a spectrum occupying the frequencies from 240 kHz up to 260…
Q: The feeder is to be used with an antenna for which an inductive reactance is measured as j60 Ohms at…
Q: Give an example of an analogue device that can be used as a phase comparator in a PLL system and…
Q: Consider the following portfolio of assets and liabilities. The time, t, is measured in years.…
Q: 1. Consider an annuity that pays £500 at the end of each year for 7 years. The annual effective…
Q: For the function f(z) = 24/(64 + 26):
i. Find the singular points of ƒ (2) and sketch them on the…
Q: A closed system containing 2 kg of air undergoes an isothermal process from 600 kPaand 200 °C to 80…
Q: Discuss at least two reasons for project delays and suggest at least two ways to avoid project…
Q: step 1: Highlight in one color the significant, non-trivial, signal words and logical (premise and…
Q: A rigid tank with a volume of 6.69 m^3 contains 15kg of saturated liquid-vapour mixture of water at…
Q: Consider
23
(x, y, z) = 10xyz - 4x²y³
v(x, y, z) = 4xy² + 3y sin(2)
Ğ(x, y, z) = Gx(x, y, z)i +…
Q: Using the method of variation of parameters, determine the
solution of the following non-homogeneous…
Q: Using the method of variation of parameters, determine the
solution of the following non-homogeneous…
Q: Using the Laplace transform, solve the following initial value
problem:
y"-4y=6e3t - 3et; y(0) = 1,…
Q: Using the following definition
00
L{f(t)} =
e-st f(t)dt
302
(0)
Obtain the Laplace transform of…
Q: Let the contour : [0,3] → C be given by
7(t) = { 11 (1) = 2eint,
0<t≤1
Y2(t) = 2(t − 2),
1<t≤3.
i.…
Q: Hi, Can you help me understand why the result of erf(0.527) is 0.5436? I understand because the…
Q: An electric motor controlling a pump draws 3.6 A from a 12 V power supply. Themechanical power…