I Review | Constants Periodic Table Part C Consider the following two expressions, the only difference being the presence or absence of parentheses. expression 1: 3x? expression 2: (3x)2 Calculate the difference between the two expressions when x 3; that is, evaluate both expressions then subtract one value from the other. Express your answer numerically. > View Available Hint(s) Submit
I Review | Constants Periodic Table Part C Consider the following two expressions, the only difference being the presence or absence of parentheses. expression 1: 3x? expression 2: (3x)2 Calculate the difference between the two expressions when x 3; that is, evaluate both expressions then subtract one value from the other. Express your answer numerically. > View Available Hint(s) Submit
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 1P: Before the element scandium was discovered in 1879, it was known as “eka-boron.” Predict the...
Related questions
Question
100%
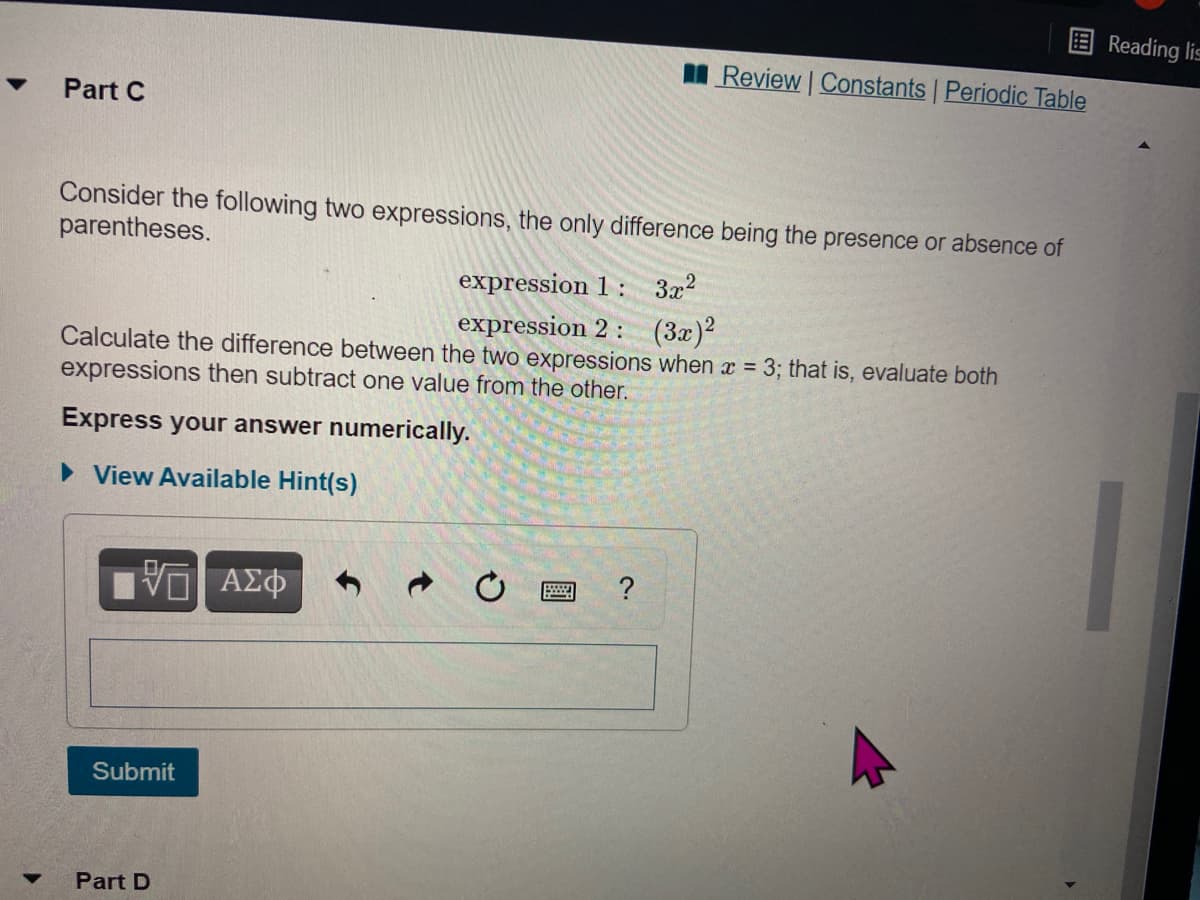
Transcribed Image Text:Reading lis
Review Constants Periodic Table
Part C
Consider the following two expressions, the only difference being the presence or absence of
parentheses.
expression 1 : 3x2
expression 2: (3x)?
Calculate the difference between the two expressions when x = 3; that is, evaluate both
expressions then subtract one value from the other.
Express your answer numerically.
• View Available Hint(s)
Submit
Part D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning