Lewis Structure (show all bonding and lone pairsi) H-N-H H. VSEPR notation The molecule is linear Arranged in a tetrahedral shape, molecule is a trigonal pyramidal H-N-H angles of 106.7° Molecular Polarity solubility in water Molecular Geometry Name
Lewis Structure (show all bonding and lone pairsi) H-N-H H. VSEPR notation The molecule is linear Arranged in a tetrahedral shape, molecule is a trigonal pyramidal H-N-H angles of 106.7° Molecular Polarity solubility in water Molecular Geometry Name
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter6: Alkanes & Alkenes
Section: Chapter Questions
Problem 3E: Using your model of butane (CH3CH2CH2CH3) , complete the following graph of the anglebetween the two...
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
Whats the answers to the last 6 blanks at the end of the table.
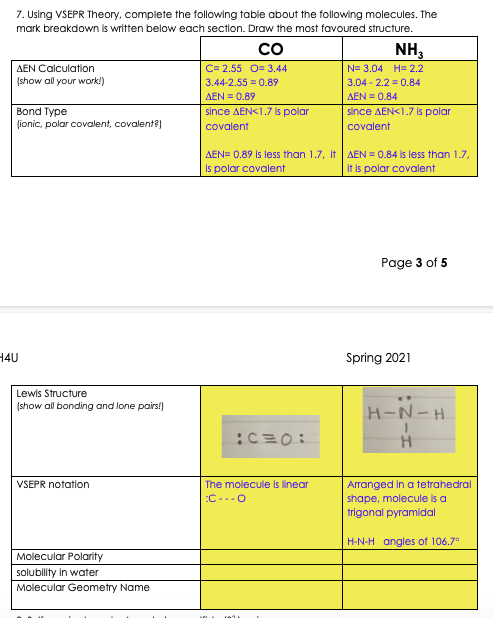
Transcribed Image Text:7. Using VSEPR Theory, complete the following table about the following molecules. The
mark breakdown is written below each section. Draw the most favoured structure.
Co
NH,
AEN Calculation
(show all your work!)
C= 2.55 O= 3.44
N= 3.04 H= 2.2
3.44-2.55 = 0.89
3.04 - 2.2 = 0.84
AEN = 0.89
AEN = 0.84
Bond Type
(ionic, polar covalent, covalent)
since AEN<1.7 is polar
since AEN<1.7 Is polar
covalent
covalent
AEN= 0.89 Is less than 1.7, It AEN = 0.84 is less than 1.7,
Is polar covalent
it is polar covalent
Page 3 of 5
14U
Spring 2021
Lewis Structure
(show all bonding and lone pairs!)
H-N-H
Arranged in a tetrahedral
shape, molecule is a
trigonal pyramidal
VSEPR notation
The molecule Is linear
:C-.-O
H-N-H angles of 106.7°
Molecular Polarity
solubility in water
Molecular Geometry Name
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
