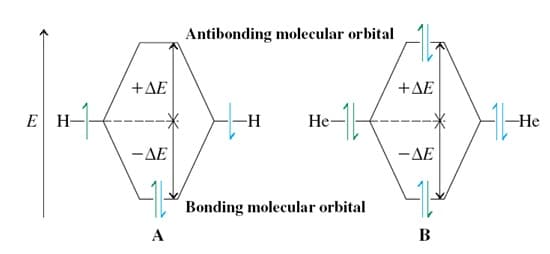
In reference to the following figure, which of the following statements is true?
a. An energy level diagram can be made of the two overlapping 1s orbitals, and the Aufbau process used to determine the electronic configurations of H2 and He2.
b. In-phase overlap of electron waves represented by the two 1s orbitals results in a new orbital having lower energy than either of the s orbitals. This new bonding orbital concentrates the electron probability between the two nuclei.
c. Out-of-phase overlap between two 1s orbitals results in a new orbital having higher energy than either of the s orbitals. This new antibonding orbital would place most of the electron probability to the left and right of the two nuclei.
d. The energy-lowering of the bonding molecular orbital and energy-raising of the antibonding molecular orbital with respect to the atomic orbitals will result in equal energy splitting with no net bonding between helium atoms.
e. All of the above statements are true.
Step by step
Solved in 2 steps


