Large 2s-2p interaction Small 2s-2p interaction B2 C2 N2 O2 F2 Nez oi, 1 1 1L 11 T2p 1 1 11 11 T2p 11 11 11 ož, 1L 1L 11 1L 11 11 o2s Bond order 2 3 2 Bond enthalpy (kJ/mol) Bond length (Å) Magnetic behavior 290 620 941 495 155 1.59 1.31 1.10 1.21 1.43 Paramagnetic Diamagnetic Diamagnetic Paramagnetic Diamagnetic A Figure 9.43 Molecular orbital electron configurations and some experimental data for period 2 diatomic molecules. Energy • |||
Large 2s-2p interaction Small 2s-2p interaction B2 C2 N2 O2 F2 Nez oi, 1 1 1L 11 T2p 1 1 11 11 T2p 11 11 11 ož, 1L 1L 11 1L 11 11 o2s Bond order 2 3 2 Bond enthalpy (kJ/mol) Bond length (Å) Magnetic behavior 290 620 941 495 155 1.59 1.31 1.10 1.21 1.43 Paramagnetic Diamagnetic Diamagnetic Paramagnetic Diamagnetic A Figure 9.43 Molecular orbital electron configurations and some experimental data for period 2 diatomic molecules. Energy • |||
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter29: Supercritical Fluid Chromatography And Extraction
Section: Chapter Questions
Problem 29.11QAP
Related questions
Question
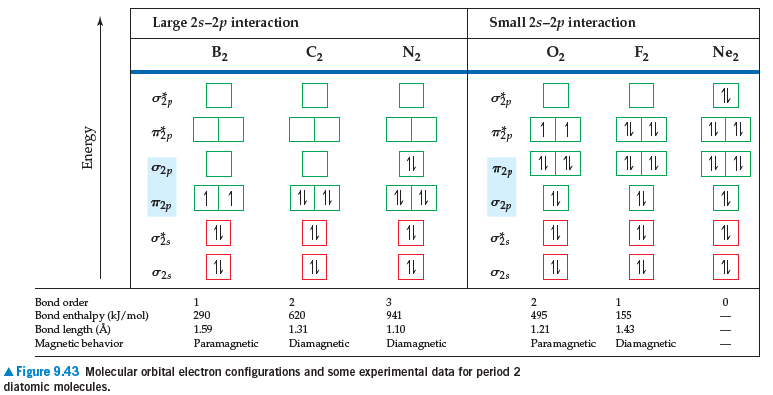
If we assume that the energy-level diagrams for homonuclear
diatomic molecules shown in Figure 9.43 can be applied
to heteronuclear diatomic molecules and ions, predict the bond order and magnetic behavior of (a) CO+, (b) NO-,
(c) OF+, (d) NeF+.
Transcribed Image Text:Large 2s-2p interaction
Small 2s-2p interaction
B2
C2
N2
O2
F2
Nez
oi,
1 1
1L 11
T2p
1 1
11 11
T2p
11
11
11
ož,
1L
1L
11
1L
11
11
o2s
Bond order
2
3
2
Bond enthalpy (kJ/mol)
Bond length (Å)
Magnetic behavior
290
620
941
495
155
1.59
1.31
1.10
1.21
1.43
Paramagnetic
Diamagnetic
Diamagnetic
Paramagnetic
Diamagnetic
A Figure 9.43 Molecular orbital electron configurations and some experimental data for period 2
diatomic molecules.
Energy
• |||
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 12 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning