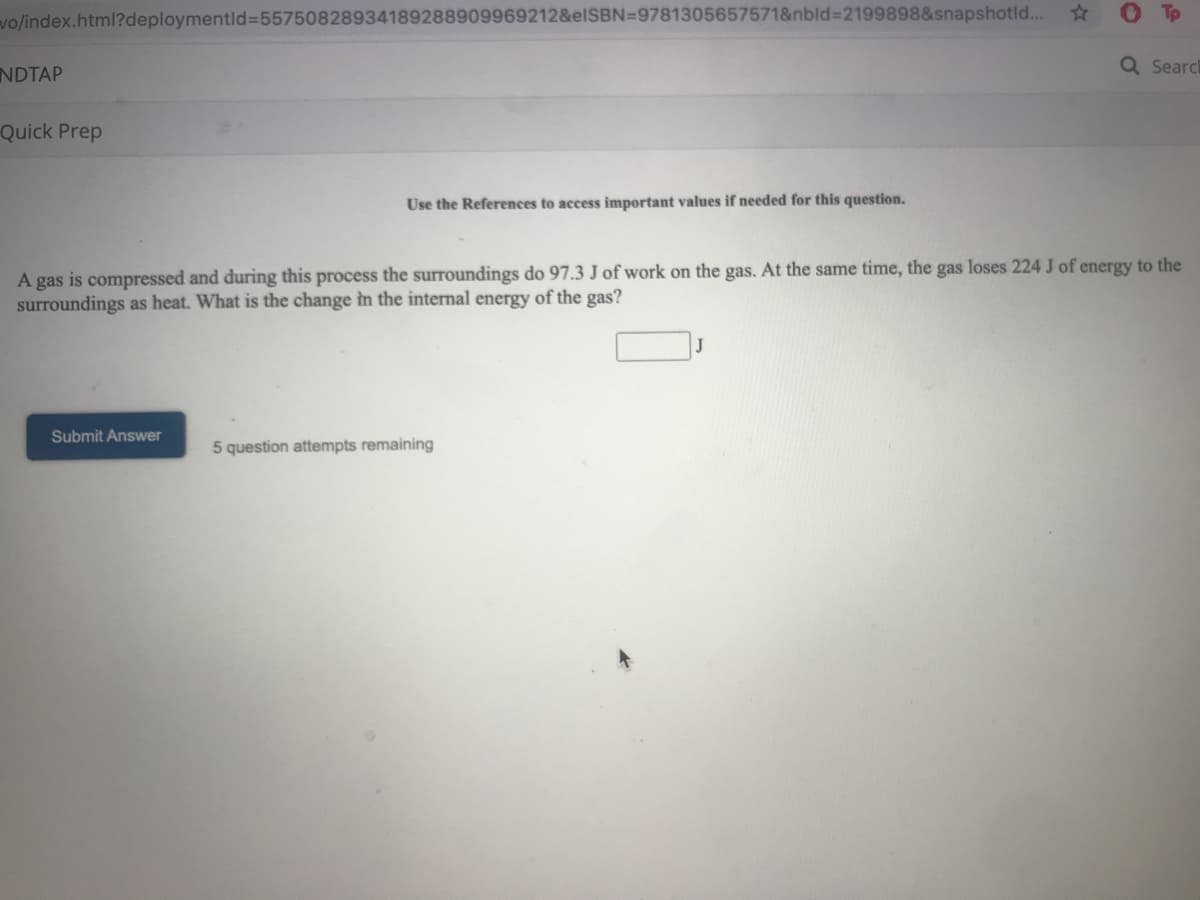
ick Prep Use the References to access important values if needed for this question. -gas is compressed and during this process the surroundings do 97.3 J of work on the gas. At the same time, the gas loses 224 J of energy to the urroundings as heat. What is the change in the internal energy of the gas?
ick Prep Use the References to access important values if needed for this question. -gas is compressed and during this process the surroundings do 97.3 J of work on the gas. At the same time, the gas loses 224 J of energy to the urroundings as heat. What is the change in the internal energy of the gas?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 155CP: Methane (CH4) gas flows into a combustion chamber at a rate of 200. L/min at 1.50 atm and ambient...
Related questions
Question
100%
Transcribed Image Text:vo/index.html?deploymentld%355750828934189288909969212&elSBN=9781305657571&nbld%3D2199898&snapshotld...
NDTAP
Q Searc
Quick Prep
Use the References to access important values if needed for this question.
A gas is compressed and during this process the surroundings do 97.3 J of work on the gas. At the same time, the gas loses 224 J of energy to the
surroundings as heat. What is the change in the internal energy of the gas?
Submit Answer
5 question attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
