Identify the characteristics of intermolecular interactions including hydrogen bonding, ion-dipole, and the hydrophobic effect. A solid line represents a covalent bond and a dottd line represents an intermolecular attraction. Sclect the image or images that represent a hydrogen bond. -O----H-C- -N----H–0- H-H ----H-F- A water molecule has polar 0–H bonds that result in regions of partial positive charge (hydrogen atoms) and a region of partial negative charge (oxygen atom with lone pains). Place the Na* and CT¯ ions where H,O molecules are properly oriented to form ion-dipole interactions. Awer Bank Select the three true statements. O Forming an ordered network of water around hydrophobic molecules increases the entropy of water. The tendency of hydrophobic molecules to aggregate in water is called the hydrophobic effect. Placing a hydrophobic molecule into water causes water molecules to orient themselves around it. Nonpolar molecules that have no polar groups (e.g. hydrocarbons) can readily form micelles. O The bilayer of a cellular membrane is primarily composed of amphipathic (amphiphilic) lipids. O O
Identify the characteristics of intermolecular interactions including hydrogen bonding, ion-dipole, and the hydrophobic effect. A solid line represents a covalent bond and a dottd line represents an intermolecular attraction. Sclect the image or images that represent a hydrogen bond. -O----H-C- -N----H–0- H-H ----H-F- A water molecule has polar 0–H bonds that result in regions of partial positive charge (hydrogen atoms) and a region of partial negative charge (oxygen atom with lone pains). Place the Na* and CT¯ ions where H,O molecules are properly oriented to form ion-dipole interactions. Awer Bank Select the three true statements. O Forming an ordered network of water around hydrophobic molecules increases the entropy of water. The tendency of hydrophobic molecules to aggregate in water is called the hydrophobic effect. Placing a hydrophobic molecule into water causes water molecules to orient themselves around it. Nonpolar molecules that have no polar groups (e.g. hydrocarbons) can readily form micelles. O The bilayer of a cellular membrane is primarily composed of amphipathic (amphiphilic) lipids. O O
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.87QE
Related questions
Question
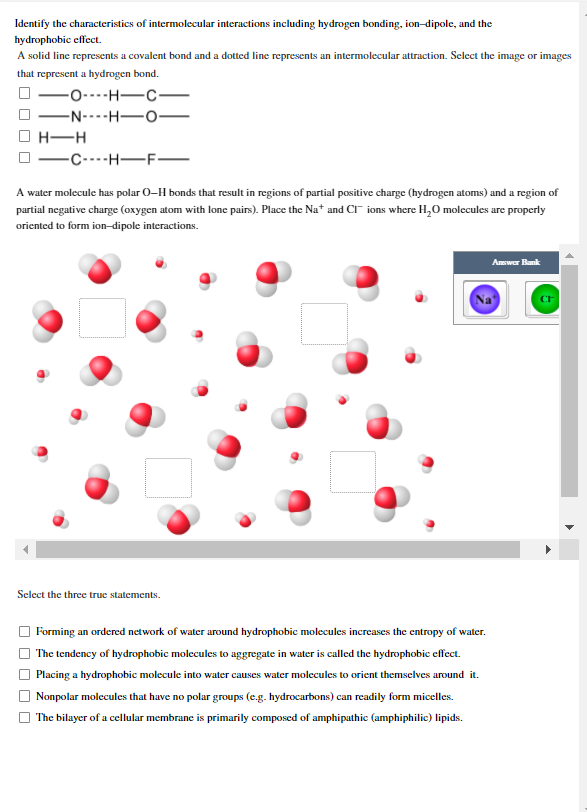
Transcribed Image Text:Identify the characteristics of intermolecular interactions including hydrogen bonding, ion-dipole, and the
hydrophobic effect.
A solid line represents a covalent bond and a dotted line represents an intermolecular attraction. Select the image or images
that represent a hydrogen bond.
-o----H-C-
-N----H-O
-
H-H
-C----H-F-
A water molecule has polar O-H bonds that result in regions of partial pasitive charge (hydrogen atoms) and a region of
partial negative charge (oxygen atom with lone pains). Place the Na* and CI ions where H,0 molecules are properly
oriented to form ion-dipole interactions.
Answer Bank
Select the three true statements.
Forming an ordered network of water around hydrophobic molecules increases the entropy of water.
The tendency of hydrophobic molecules to aggregate in water is called the hydrophobic effect.
Placing a hydrophobic molecule into water causes water molecules to orient themselves around it.
Nonpolar molecules that have no polar groups (e.g. hydrocarbons) can readily form micelles.
The bilayer of a cellular membranc is primarily composed of amphipathic (amphiphilic) lipids.
O O O O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning