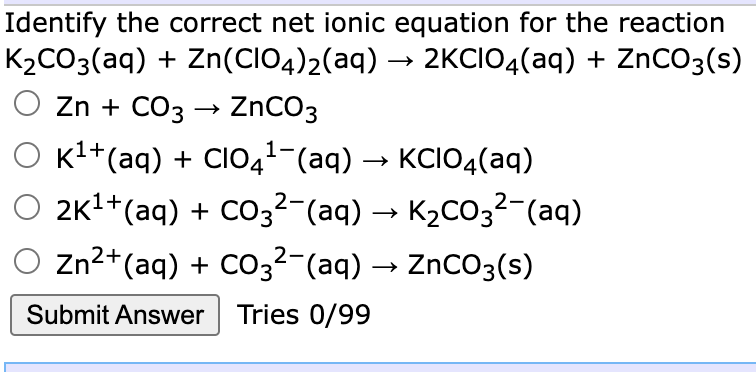
Identify the correct net ionic equation for the reaction K2CO3(aq) + Zn(CIO4)2(aq) → 2KCIO4(aq) + ZnCO3(s) Zn + CO3 → ZnCO3 Kl+(aq) + CIO41-(aq) → KCIO4(aq) O 2k1+(aq) + CO,²-(aq) → K2CO3²-(aq) O zn2+(aq) + Co32-(aq) → ZnCO3(s)
Q: Write the net ionic equation, including phases, that corresponds to the reaction…
A:
Q: Predict the products of the following reaction and balance the equation: __H2SO4(aq) + __BaCl2(aq)→…
A:
Q: What is the net ionic equation for the reaction in the Fourth Well with Fe(NO3)3 and KOH? O Fe(NO3)3…
A:
Q: The net ionic equation below represents the standardization of potassium permanganate. 5 H2O2 (1) +…
A:
Q: For the analysis of trace metals it is necessary to prepare solutions of very low concentrations…
A: To solve this question we use the total volume strength formula.
Q: Given the type for each of the following reactions, predict the products and then balance them. If a…
A: Since you have posted question with multiple sub-parts, we are entitled to answer the first 3 only.…
Q: A chemist performs a gravimetric analysis. The chemist combines 1.00L of 2.00MAgNO3(aq) with 1.00L…
A: Double displacement reaction A chemical reaction is said to be a double displacement reaction when…
Q: O A chemical engineer determines the mass percent of iron in an ore sample by converting Fe to Fe in…
A: The balanced reaction taking place is given as, Given : Concentration of KMnO4 = 0.03190 M Volume…
Q: Which equation below is the balanced total ionic equation for the reaction of potassium chloride…
A: Reaction of pottasium chloride with Lead (II) Nitrate Pb(NO3)2 + KCl --> KNO3 + PbCl2
Q: Balance it using the ion-electron method. O 2AI(s) + Cu²*(aq) → 2A13+(aq) + 3 Cu(s) O Al(s) +3…
A:
Q: Identify the oxidizing agent and the reducing agent in the following reaction, and explain your…
A: Identify the oxidizing agent and the reducing agent in the following reaction, and explain your…
Q: The iron content of a mineral deposit was determined by titration with KMNO4 to see if it had enough…
A:
Q: Bromine (Br2) reacts with sulfurous acid (H2SO3), producing dithionate ions (S206) and bromide ions,…
A:
Q: What is the net ionic equation for the reaction of AgNO3(aq) with K2SO4(aq). Include phases.
A: The net ionic equation shows only the chemical species that are involved in a reaction, while the…
Q: Complete and balance the following equation: Cr2O2−7(aq)+CH3OH(aq)→HCO2H(aq)+Cr3+(aq) (acidic…
A: The given redox reaction is as follows: Cr2O72- + CH3OH → HCO2H + Cr3+ A redox reaction is balanced…
Q: Balance it using the ion-electron method. O 2Al(s) + Cu²*(aq) → 2A13+(aq) + 3 Cu(s) O Al(s) + 3…
A: This is a redox reaction. The basic reaction will be :- Al + Cu2+ → Al3+ + Cu
Q: The oxidizing agent in the reaction below is 3CuCl2(aq)+2Al(s) →3Cu(s)+2AlCl3(aq) Cu2+ Al Cu…
A:
Q: A 0.7785 g sample containing hydrazine (N2H4, rocket fuel) was dissolved and diluted to 250.0 mL in…
A: Answer: This question is based on stoichiometric calculation where we have to convert the moles of…
Q: The iron content of a mineral deposit was determined by titration with KMNO4 to see if it had enough…
A:
Q: 4. In the following reaction, what substance loses electrons? 2 Fe (aq) + Sn2 (aq) → 2 Fe2"(aq) +…
A:
Q: Given the reaction: 2HNO3 + Cu + H+ à 2NO2 + Cu2+ + 2H2O. Which statement is correct? A) N is the…
A: Since you have posted multiple question. We will solve the first question for you. To get the…
Q: n the textile industry, chlorine is used to bleach fabrics. Any of the toxic chlorine hat remains…
A: Given values -> Weight of Na2S2O3 = 146.66kg =146660gram Weight of Cl2 = 42.09 kg = 42090gram
Q: (ii) A student was given 400 cm of aqueous ammonia solution, NH, (aq). The student was asked to…
A: The balanced reaction given is: 2NH3(aq)+H2SO4(aq)→(NH4)2SO4 Conversion unit: 1 L=1 dm31 L=1000 cm3…
Q: Which process could not be an electrolytic reaction? a. 2 PbSO4(s) + 4 H2O(l) Pb(s) +…
A:
Q: Which equation below is the balanced net ionic equation for the reaction of potassium chloride with…
A: Given, the reaction between potassium chloride and lead(II) nitrate.we are asked to write the net…
Q: In the reaction Pb(s) + 2Ag* (aq)Pb2+ (aq) + 2Ag(s) -> (a) Which species is oxidized and which is…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: A sample of soda ash (impure Na2CO3) is titrated with 0.5000 N H2SO4. If the sample weighs 1.1000 g…
A:
Q: Iron(III) was reduced to iron(II) by chromium(III) in acidic solution according to the following…
A: The oxidation state of chromium in chromium oxide product and possible chromium oxide product has to…
Q: Breathalyzers determine the alcohol content in a person's breath by the following (unbalanced) redox…
A: Seven steps are required to balance the given redox reaction in acid solution. First reduction and…
Q: Potassium permanganate in acidic solution is used to titrate a solution of iron(II) ions, with which…
A:
Q: 1) The net ionic equation for formation of an aqueous solution of Nil2 accompanied by evolution or…
A: Given, When aqueous solution of NiCO3 react with aqueous HI to form solid NiI2 and CO2 gas the net…
Q: In the following reaction which occurs from left to right: 2 Fe3+(aq) + Zn(s) → 2 Fe2*(aq) +…
A: The substance which undergoes oxidation is reducing agent and substance which undergoes reduction is…
Q: Write the molecular equation for the reaction of iron(1II) bromide with potassium carbonate. A)…
A: FeBr3 is Iron (III) bromide K2CO3 is Potassium carbonate FeBr3 has Fe3+ and Br- ions K2CO3 has K+…
Q: 3. . Predict the products and states of the products for the following reactions. Balance the…
A: The reaction for the following reaction is as Follows: a) H2SO4 (aq) + MgCO3 (s) →MgSO4 (aq) + CO2…
Q: 2 MnO4- (aq) + 5 C2O42- (aq) + 16 H+ (aq) « 2Mn2+ (aq) + 10 CO2 (g) + 8 H2O(l) 0.4040 g…
A: The balanced Chemical Reaction given as - 2 MnO4- (aq) + 5 C2O42- (aq) + 16 H+ (aq) --->…
Q: Hello, can this question please be explained. Determine the products of the following reaction…
A: Given, The products of the following reaction is: K2CO3 (aq) + NiF2 (aq) →
Q: 15. Oxidizing agent of reaction in acidic Cr,03 (aq) +1 (aq) Cr3+ (aq) + I2(s)
A: Note: As per our guidelines, we are supposed to answer only one question when multiple questions are…
Q: Decide whether each chemical reaction in the table below is an oxidation-reduction ("redox")…
A:
Q: In the reaction Zn(s) + Cu2*(aq) Zn2*(aq) + Cu(s), O Cu2*(aq) is the oxidizing agent and Zn(s) is…
A:
Q: Sn2+ can be analysed by reaction with excess Fe3+ and the Fe2+ produced is oxidised back to Fe3+…
A: Here we are required to find the moles of Sn2+ present which was analysed by redox titration using…
Q: II. Identify oxidizing agent and reducing agent in the reactions. 1. 2Na,S,O3 + 2→ Na,S,O6 + 2Nal 2.…
A:
Q: 33 On the following reactions: MnO4− + I− → MnO4− + IO4−, which is the oxidizing agent? Select one:…
A: MnO4- + I- → MnO2 + IO4- In this given recation, MnO-4 is reduced and I- is oxidised.
Q: The blood alcohol (C2H5OH) level can be determined by titrating a sample of blood plasma with an…
A: 0.08 % blood alcohol limit means that the maximum permissible alcohol in blood is 0.08 g per 100 mL…
Q: In the following reaction, identify the oxidizing agent. Cu(NO3)2 (aq) + 2 V(NO3)2 (aq) Cu (s) + 2…
A: B. Cu2+
Q: The neutralization reaction between H2SO4 and LiOH produces the salt with the formula ________.…
A: A neutralization reaction can be defined as a chemical reaction between a base and an acid to form a…
Q: Unbalanced: Fe²*(aq) + Cr2O;²(aq) → Fe*(aq) + Cr³*(aq) Identify the following statements as true or…
A:
Q: Dichromate reacts with aqueous iron (II) ion in acidic solution according to the balanced equation:…
A: During the titration, the moles of species K2Cr2O7 and FeSO4 is equivalent then this point will be…
Q: Determine the Oxidizing and Reducing Agent in the reactions below: 3 Cl2(g) + 2 Fe(s) → 6 Cl– (aq) +…
A:
Q: What is the oxidation state of Iron (Fe) in K3[Fe(C2O4)3.3H2O?
A: Oxidation number, also called oxidation state, the total number of electrons that an atom either…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- Write the net ionic equation for the reaction of iron (III) chloride with sodium carbonate. 2Fe(aq) + 3CO3(aq) ⟶⟶Fe2(CO3)3(s) 2Fe+3(aq) + 3CO3-2(aq) ⟶Fe2(CO3)3(s) Fe+3(aq) + CO3-2(aq) ⟶⟶ Fe2(CO3)3(s) 2Fe+3(aq) + CO3-2(aq) ⟶⟶ Fe2(CO3)3(s) Fe+3(aq) + 3CO3-2(aq) ⟶⟶Fe2(CO3)3(s)The following reaction occurs in basic solution: _ H2O(aq) + _ MnO4–(aq) + _ ClO–(aq) → _ MnO2(s) + _ ClO4–(aq) + _ OH–(aq) When the equation is properly balanced, what is the sum of the lowest whole-number coefficients? a. 9 b. 10 c. 20 d. 12 e. 6Balance the following reactions in acid or base as specified. Fe²⁺ + Cr₂O₇² → Fe³⁺ + Cr³⁺ (in acidic solution) CN⁺ + Fe³⁺ → CNO⁻ + Fe²⁺ (in basic solution) Cr(OH)₂ + Br₂ → CrO₄²⁺ + Br⁻ (in basic solution)
- A 25.00cm3 of potassium dichromate(K2Cr2O7) solution are added to an acidified aqueous solution of potassium iodide(present in excess). The Iodine was then liberated according to the equation K2Cr2O7(aq) + 14H+(aq) + KI(aq) → K+(aq) + Cr3+(aq) + H2O(l) + I2(aq) required 19.63cm3 of a 0.1478MNa2S2O3 for complete reaction. The equation for the reaction of between Na2S2O3 and I2 is I2 +(aq) + Na2S2SO3(aq) → Na2S4O6(aq) + NaI(aq) Calculate the concentration of potassium dichromate (only 4 decimal places). ________Mol/LThe blood alcohol (C2H5OH) level can be determined by titrating a sample of blood plasma with an acidic potassium dichromate solution, resulting in the production of Cr3+ (aq) and carbon dioxide. The reaction can be monitored because the dichromate ion (Cr2O72-) is orange in solution, and the Cr3+ ion is green. The balanced equation is 16H+(aq) + 2Cr2O72-(aq) + C2H5OH(aq) -> 4Cr3+(aq) + 2CO2(g) + 11H20. If the blood alcohol limit is 0.08%, what concentration in M of Cr2O72-(aq) would you use , such that titrant volume will be 2.00 mL for a 5.00 mL sample that would be over the limit ? Specify what change you will monitor at equivalence point.Oxalic acid (H2C2O4) is present in many plants and vegetables. Balance the following equation in acid solution: MnO4- + C2O4- → Mn2+ + CO2
- Consider the following reactions. reaction (1): Mn2+(aq) + SO42−(aq) → MnO4−(aq) + H2SO3(aq) in acidic solutionreaction (2): Ni2+(aq) + Cl−(aq) → Ni(s) + ClO−(aq) in basic solutionreaction (3): Mo(s) + N2O4(g) → MoO2(s) + HNO2(aq) in acidic solutionreaction (4): MnO4−(aq) + Cl−(aq) → MnO2(s) + ClO3−(aq) in basic solution Balance each equation under the specified conditions. (Include states-of-matter under the given conditions in your answer.)reaction (1): Mn2+(aq) + SO42−(aq) → MnO4−(aq) + H2SO3(aq) in acidic solution chemPad Help reaction (2): Ni2+(aq) + Cl−(aq) → Ni(s) + ClO−(aq) in basic solution chemPad Help reaction (3): Mo(s) + N2O4(g) → MoO2(s) + HNO2(aq) in acidic solution chemPad Help reaction (4): MnO4−(aq) + Cl−(aq) → MnO2(s) + ClO3−(aq) in basic solution chemPad HelpPotassium permanganate solutions used in oxidation-reduction titrations are often standardized against sodium oxalate, Na₂C₂O₄, used as a primary standard. The reaction involved is 5C₂O₄²⁻(aq) + 2MnO₄⁻(aq) + 16 H⁺(aq) → 2Mn²⁺(aq) + 8H₂O(l) + 10CO₂(g) A 0.2452 g sample of sodium oxalate is dissolved in 100 mL of acid solution, and the permanganate solution is added slowly from a buret. The endpoint is reached when 15.85 mL of the permanganate solution has been added. The molar mass of sodium oxalate is 134.00 g/mol. Calculate [MnO₄⁻], the molar concentration of the permanganate solution.Which of the following reactions will occur spontaneously as written? 2Cr3+ (aq) + 3Sn2+ (aq) → 3Sn4+ (aq) + 2Cr (s) Sn4+ (aq) + Fe2+ (s) → Sn2+ (aq) + Fe (s) 3Fe2+ (aq) + Cr3+ (aq) → Cr (s) + 3Fe3+ (aq) 2Cr (s) + 3Fe2+ (s) → 3Fe (s) + 2Cr3+ (aq
- The amount of I3- (aq) in a solution can be determined by titration with a solution containing a known concentration of S2O32- (aq) (thiosulfate ion). The determination is based on the net ionic equation 2S2O32- (aq) + I3- (aq) yields to S4O62- (aq) + 3I- (aq) Given that it requires 34.2 mL of 0.500 M Na2S2O3 (aq) to titrate a 20.0 mL sample of I3- (aq), calculate the molarity of I3- (aq) in the solution.Identify the best oxidizing agent Zn2+ + 2e- ↔ Zn Eo = -0.76 V Cu2+ + 2e- ↔ Cu Eo = +0.34 V Cr2O72- + 14H+ + 6e- ↔ 2Cr3+ + 7H2O Eo = +1.33 V a. Cu2+ b. Cr3+ c. Zn 2+ d. Cr2O72-Balance the following in acidic solution:(a) H2 O2 + Sn2+ ⟶ H2 O + Sn4+(b) PbO2 + Hg ⟶ Hg22+ + Pb2+(c) Al + Cr2 O72− ⟶ Al3+ + Cr3+

