Untitled + X tml?courseld=15539865&HeplD=676f177415469ec3dc78ff3c8bf724d9#10001 nalized Invit... The Corinthian Ho... Wholesale Glass... Shop | Origami Owl O Inbox Outlook W... Sugarpill Cosmeti... 4 HW ation Electron Configurations 11 of 13 Consider that a single box represents an orbital, and an electron is represented as a half arrow. Orbitals of equal energy are grouped together. Sort the various electron configurations based on whether they are valid, whether they break Hund's rule, or whether they break the Pauli exclusion principle. The electron configuration will be valid if it follows both Hund's rule and the Pauli principle. Drag the appropriate items to their respective bins. View Available Hint(s) Help Reset 1 1 1 2p 11 2p 1111 1. 2p 1s 2s 2p 1s 1s 2s 1s 2s 1s 1s 2s 1s 2s 2p 1s 2s Breaks the Pauli principle Breaks Hund's rule Valid PPearson Permissions Contact Us f LUco LPrivacy Policv
Untitled + X tml?courseld=15539865&HeplD=676f177415469ec3dc78ff3c8bf724d9#10001 nalized Invit... The Corinthian Ho... Wholesale Glass... Shop | Origami Owl O Inbox Outlook W... Sugarpill Cosmeti... 4 HW ation Electron Configurations 11 of 13 Consider that a single box represents an orbital, and an electron is represented as a half arrow. Orbitals of equal energy are grouped together. Sort the various electron configurations based on whether they are valid, whether they break Hund's rule, or whether they break the Pauli exclusion principle. The electron configuration will be valid if it follows both Hund's rule and the Pauli principle. Drag the appropriate items to their respective bins. View Available Hint(s) Help Reset 1 1 1 2p 11 2p 1111 1. 2p 1s 2s 2p 1s 1s 2s 1s 2s 1s 1s 2s 1s 2s 2p 1s 2s Breaks the Pauli principle Breaks Hund's rule Valid PPearson Permissions Contact Us f LUco LPrivacy Policv
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 6P: Using Table 5.2, write down the mathematical expression for the 2px wave function for an...
Related questions
Question
Transcribed Image Text:Untitled
+
X
tml?courseld=15539865&HeplD=676f177415469ec3dc78ff3c8bf724d9#10001
nalized Invit...
The Corinthian Ho...
Wholesale Glass...
Shop | Origami Owl
O Inbox Outlook W...
Sugarpill Cosmeti...
4 HW
ation Electron Configurations
11 of 13
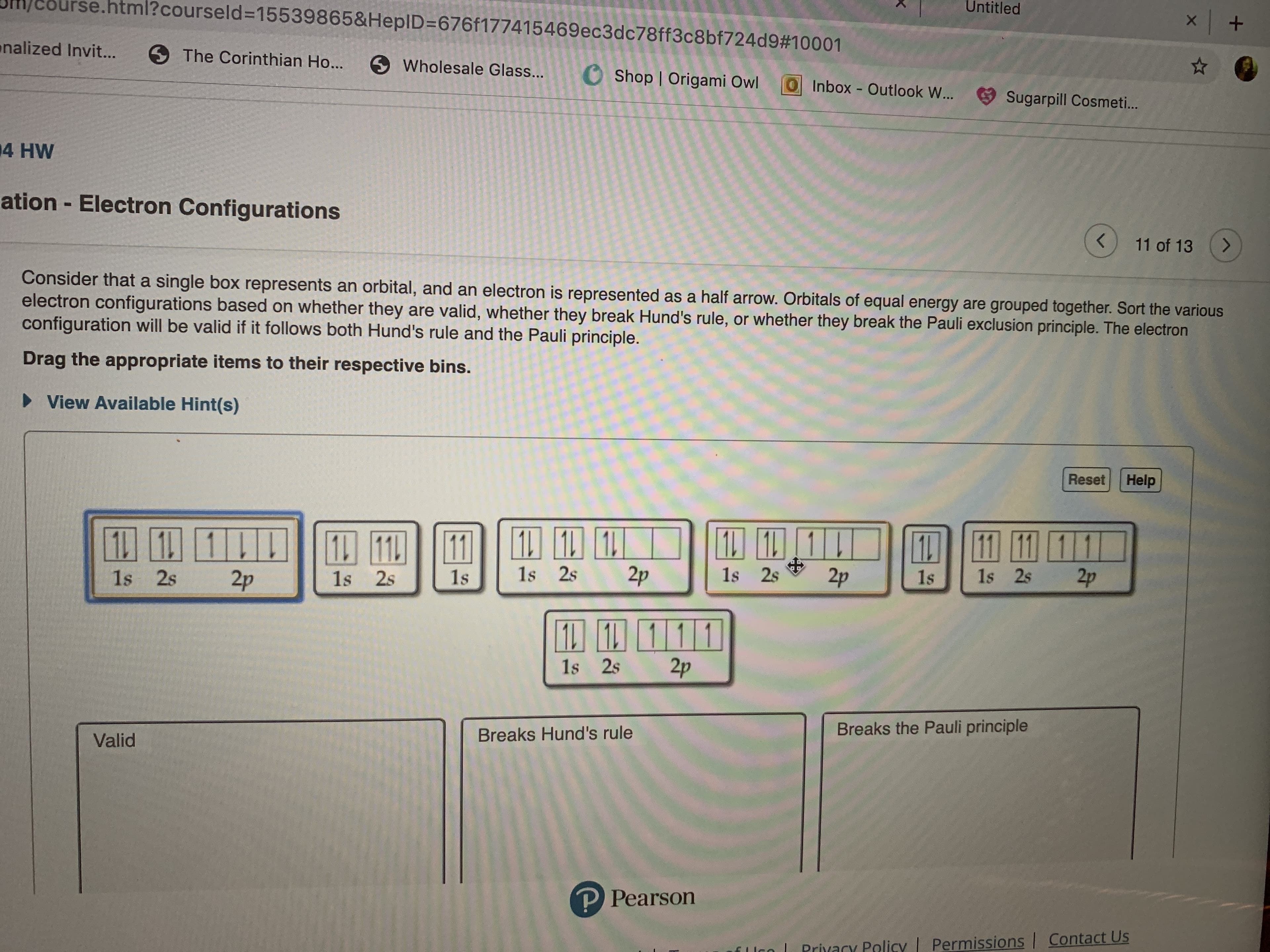
Consider that a single box represents an orbital, and an electron is represented as a half arrow. Orbitals of equal energy are grouped together. Sort the various
electron configurations based on whether they are valid, whether they break Hund's rule, or whether they break the Pauli exclusion principle. The electron
configuration will be valid if it follows both Hund's rule and the Pauli principle.
Drag the appropriate items to their respective bins.
View Available Hint(s)
Help
Reset
1
1 1
2p
11
2p
1111
1.
2p
1s 2s
2p
1s
1s 2s
1s 2s
1s
1s 2s
1s 2s
2p
1s 2s
Breaks the Pauli principle
Breaks Hund's rule
Valid
PPearson
Permissions Contact Us
f LUco LPrivacy Policv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
