Identify the species oxidized, the species reduced, the oxidizing agent and the reducing agent in the following electron transfer reaction. Hg²+ + Co- Hg + Co²+ species oxidized species reduced oxidizing agent reducing agent As the reaction proceeds, electrons are transferred from to
Identify the species oxidized, the species reduced, the oxidizing agent and the reducing agent in the following electron transfer reaction. Hg²+ + Co- Hg + Co²+ species oxidized species reduced oxidizing agent reducing agent As the reaction proceeds, electrons are transferred from to
Chapter5: Chemical Reactions
Section: Chapter Questions
Problem 5.78E
Related questions
Question
Help
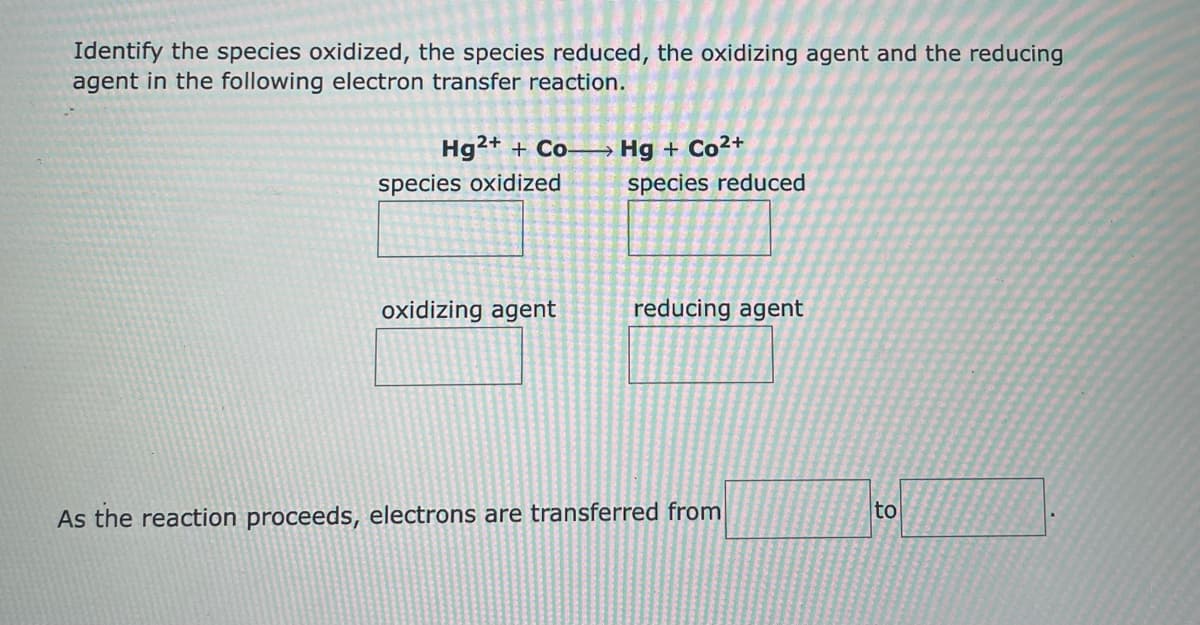
Transcribed Image Text:Identify the species oxidized, the species reduced, the oxidizing agent and the reducing
agent in the following electron transfer reaction.
Hg²+ + Co-
Hg + Co²+
species reduced
species oxidized
oxidizing agent
reducing agent
As the reaction proceeds, electrons are transferred from
to
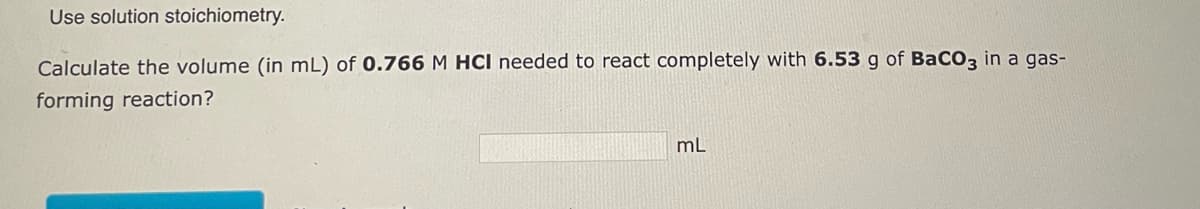
Transcribed Image Text:Use solution stoichiometry.
Calculate the volume (in mL) of 0.766 M HCI needed to react completely with 6.53 g of BaCO3 in a gas-
forming reaction?
mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning