If 1 gram of carbon black was used in the experiment, and the area occupied by one nitrogen molecule to be 16.2r10 -20 m² , calculate the surface area in square meters with k=4.05 by the Harkins – Jura method. ww w w ww w
If 1 gram of carbon black was used in the experiment, and the area occupied by one nitrogen molecule to be 16.2r10 -20 m² , calculate the surface area in square meters with k=4.05 by the Harkins – Jura method. ww w w ww w
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter22: Surfaces
Section: Chapter Questions
Problem 22.46E: Early attempts to coat metals with Teflon, poly tetrafluoroethylene, resulted in a polymer layer...
Related questions
Question
Thank you in advance!
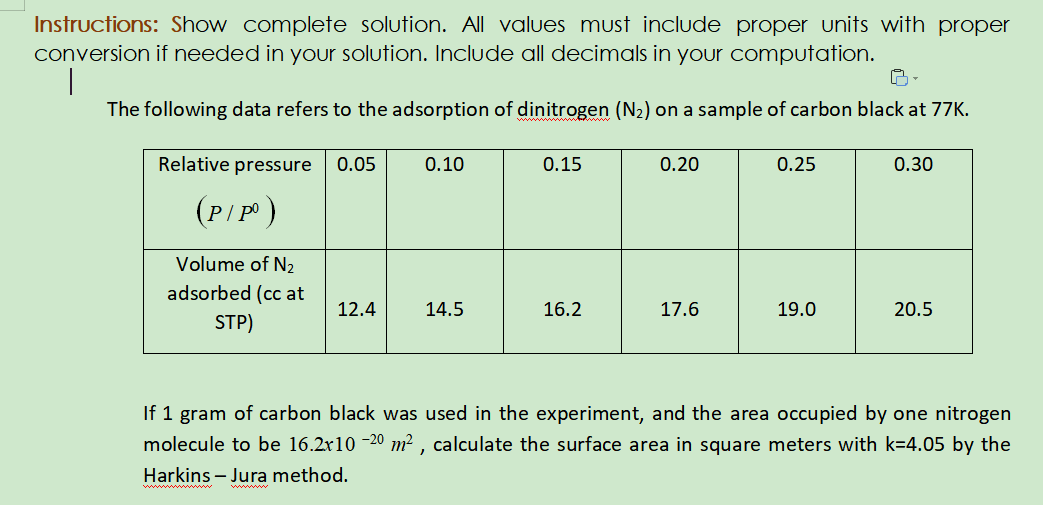
Transcribed Image Text:Instructions: Show complete solution. All values must include proper units with proper
conversion if needed in your solution. Include all decimals in your computation.
The following data refers to the adsorption of dinitrogen (N2) on a sample of carbon black at 77K.
Relative pressure
0.05
0.10
0.15
0.20
0.25
0.30
(P/P)
Volume of N2
adsorbed (cc at
12.4
14.5
16.2
17.6
19.0
20.5
STP)
If 1 gram of carbon black was used in the experiment, and the area occupied by one nitrogen
molecule to be 16.2r10 -20 m² , calculate the surface area in square meters with k=4.05 by the
Harkins – Jura method.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,
