If 7.5 g of KBr were dissolved in 100. g of water, how much heat (kJ) was involved? (2 Significant Figures) kJ J (q = msolution*C*AT where the heat capacity of the solution is 4.18
Q: If the specific heat of a solution is 4.18 J/goC, and you have 161 mL (1.03 g/mL) which increases in…
A: Mass of solution in grams is determined as follows,
Q: What is the specific heat (J/g∙°C) of a metal object whose temperature increases by 3.0°C when 17.5…
A: The specific heat capacity (c) in J/goC is calculated as shown below where Q, m, and ΔT are the…
Q: If 1101 J of heat is available, what is the mass in grams of iron (specific heat = 0.450 J/g•°C)…
A: HEAT ENERGY: When the atoms present in a compound which can be a solid, liquid or a gaseous molecule…
Q: 4 The specific heat of wood is 2.03 Jig C. How much heat is released when 550 g of wood is cooled…
A: q= m.c.∆T q =heat=? m=mass =550 g C=specific heat= 2.03 j/g℃ ∆T=difference in temperature =140℃
Q: What is the specific heat of a metal whose mass is 94.5 g if the temperature changes from 250°C to…
A: It is given that the mass of the metal is 94.5 g and is undergoing a temperature change from 250°C…
Q: 2. Thirty-two joules of heat is added to a 255 gram iron bar How much will the iron bar's…
A:
Q: If it takes 37.0 cal of heat to raise the temperature of 12.0 g of a substance by 8.5 °C, what is…
A: The amount of heat required to raise the temperature of a substance through 1oC is known as the…
Q: What is the specific heat of tin if a 55.0-g sample absorbs 982 J of heat to raise its temperature…
A:
Q: A work of 657 J is done on a system that releases 434 J of heat. What is the energy change in the…
A: According to first law of thermodynamics, internal energy of the system is equal to the sum of work…
Q: 24.0 g of metal at 78.3°C is cooled to 12.4°C and produces 194 J of heat. What is the specific heat…
A: Mass of substance M = 24.0 g Initial temperature = 78.3 °C Final temperature = 12.4°C Heat…
Q: Methane, CH4, is a major component of marsh gas. When 0.5000 mol methane burns to produce carbon…
A: Given : Heat = -445.1 KJ
Q: 2. What is the specific heat of a metal whose mass is 94.5 g and the temperature changes from 48 °C…
A: Consider the given information is as follows; Mass of the metal = 94.5 g Initial temperature T1…
Q: If the specific heat of water is 4.186 J/g °C, how much heat is required to increase the temperature…
A:
Q: How much heat in kilojoules is released when 209.0 g hydrogen bromide (HBr) is dissolved if its…
A: Given: Molar heat of solution = -85.1 kJ/mol Mass of HBr = 209.0 g Known: Molar mass of HBr = 80.91…
Q: The amount of heat absorbed by the solution can be found using the equation q = m x SH x AT. It can…
A: A multiple choice question based on heat absorbed, which is to be accomplished.
Q: If 7.5 g of KBr were dissolved in 100. g of water, how much heat (kJ) was involved? (2 Significant…
A:
Q: What is the specific heat of 54.8 g of a substance that releases 445 Joules of heat upon cooling…
A: q = mc∆T q is the amount of heat energy released m is the mass of substance c is the specific heat…
Q: 3. a. What is the specific heat of a 41.5 g piece of glass, if it takes 17.8 kJ of heat to raise its…
A: (3a) Specific heat of a Substance can be calculated by using Following formula - Q = C M ∆T Here, Q…
Q: 100.g of Cu at 100 degrees C is added to 150.g of H2O at 10 degrees C. If the specific heat of…
A: From the question,Mass of Cu =100 g.The temperature of Cu = 100˚C.Mass of H2O = 150 g.The…
Q: 100 g of a solution (specific heat of 0.32 J/g C) undergoes a reaction in which 74.84 J of heat is…
A: Mass of solution = 100 g Specific heat = 0.32 J/g oC Heat released = 74.84 J
Q: A 22.0 g block of copper at 45'C absorbs 2.50 kJ of heat. Given the specific heat of Cu is 0.385…
A: given :- mass of Cu = 22.0 g specific heat of Cu = 0.385 J/g°C initial temperature = 45 °C heat…
Q: If 31,500J of heat is used to warm 750g of water, what is the temperature change? The specific heat…
A: According to the law of calorimetry, Heat absorbed by an object can be written as, Q = ms∆T Where,…
Q: The temperature of a beaker containing 194 mL (density of water is 1 g/mL) of water changes from 16…
A:
Q: The heat of 12 kJ in J will be?
A:
Q: Hydrogen sulfide, H2S, is produced during decomposition of organic matter. When 0.6850 mol H2S burns…
A:
Q: A solution is made by mixing 197.3 g of ethanol initially at 10.5°C with 250.0 g of water initially…
A: We know that, according to the law of conservation of heat energy, energy gained or loss by the…
Q: Given that 158.05 g of solution increased in temperature by 4.82 "C. how much heat was gained by the…
A: Given that: Mass of solution=158.05grams Change in temperature=4.82°C Specific heat of…
Q: If 1001 J of heat is available, how many grams of iron (specific heat = 0.45 J/g・°C) can be heated…
A: The equation is:
Q: f 1.005 moles of nitric acid are completely neutralized by an equivalent number of moles of sodium…
A: Given reaction : HNO3 + NaOH -------> NaNO3 + H2O ΔHrxn = -57.3 kJ/mol
Q: What is the specific heat of a substance if 373 J of heat is required to raise the temperature of a…
A: Q = m . s . ∆T Where, Q = heat required; m = mass of substance; s = specific heat and ∆T =…
Q: what is the mass in grams of iron
A:
Q: How much heat, in joules, must be added to a 95.8 g iron block with a specific heat of 0.509 J/g °C…
A:
Q: Hydrogen sulfide is produced during the decomposition of organic matter. When 0.5380 mol H2S burns…
A: Given: H2S burns to produce SO2(g) and H2O(g) with heat release of -278.7 kJ
Q: rogen sulfide, H2S, is produced during decomposition of organic matter. When 0.4190 mo H2S burns to…
A: Given, Heat released = -217.0 KJ
Q: 4. What is the specific heat (J/g °C) of a metal object whose temperature increased from 18.5°C to…
A: Given: The mass of the metal = 58.5 g The amount of heat = 109.5 J The initial temperature of the…
Q: If the heat of solution for a chemical was -22,000 cal,g when 2.0 g of solid was dissolved, the…
A: Calculation of Heat of solution.
Q: heat
A:
Q: What mass of water will change its temperature by 3.00 degrees C when 525 J of heat is added to it?
A: The answer to the following question is-
Q: The specific heat of an unknown metal is calculated to be 3.74J/g*c. What is the mass of the sample…
A: Specific heat :- The amount of heat required to rise the temperature of one gram of a substance…
Q: 1. What is the specific heat of a 50- gram metal initially at 200C which heated to 900 C with 2330 J…
A: Given: Initial temperature of metal = 200 oC Final temperature of metal = 900 oC Mass of metal = 50…
Q: When 20 grams of a salt is dissolved in 95 grams of water, the temperature of the solution decreases…
A: Given, mass = 20 grams + 95 gram = 115 gram ∆T=T2 - T1 = 30-40 =-10 oC c = 1.1cal/g oC ∆Q…
Q: If the heat of solution of a salt is highly endothermic and 100 g of that salt are dissolved in 1 L…
A: When heat of solution of a salt is highly endothermic it means that during dissolution of that salt…
Q: A dilute solution of hydrochloric acid with a mass of 606.46 g and containing 0.37376 mol of HCl was…
A:
Q: If 14.5 kJ of heat were added to 485 g of liquid water, how much would its temperature increase?
A: The increase in temperature of a substance with respect to the quantity of heat transferred depends…
Q: How much heat is required, in calories, to raise the temperature of 57.8 g of silver from 17.0C to…
A: Mass of silver (m) = 57.8 g Initial temperature (T1) = 17.0°C Final temperature (T2) = 43.5°C…
Q: The specific heat of water is 4.184J/g°c. How many joules of heat will be removed from 2.00g of…
A: The amount of heat absorbed or released is related to specific heat capacity as,…
Q: A total of 54.0 Joules of heat are observed as 58.3g of lead is heated from 12.0°C to 42.0°C. From…
A:
Q: Hydrogen sulfide, H2S, is produced during decomposition of organic matter. When 0.3620 mol H2S burns…
A: Given :- H2S (g) + O2 (g) → SO2 (g) + H2O (g) ∆H = -187.5 kJ To calculate :- Given heat into…
Q: Question 3
A: Given,Mass of barium chloride = 25gMass of water = 75gCalorimeter constant = 120J/degree Celsius.
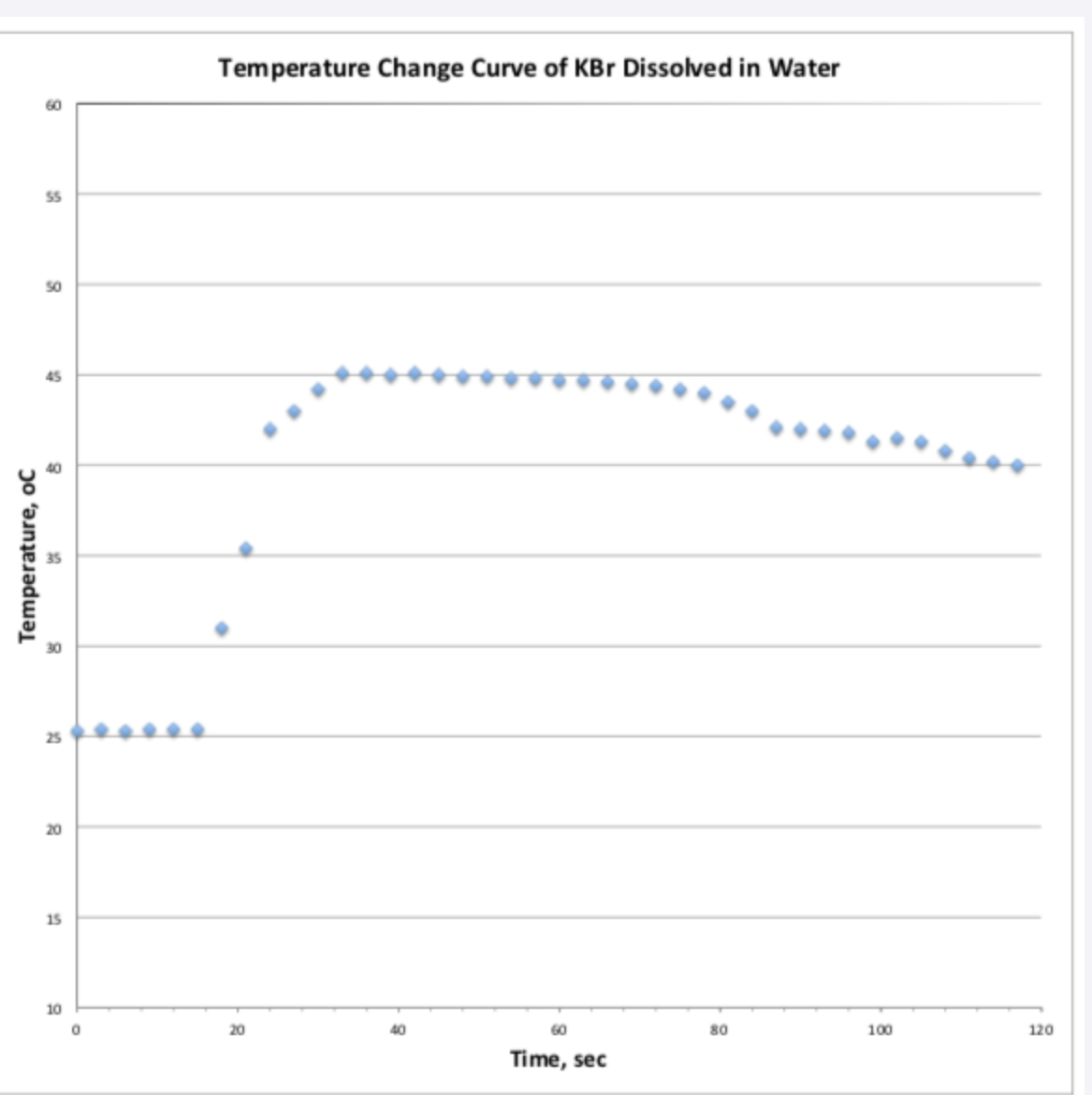
Ti is 25C
I do not know what Tf is because I put 40 and it was wrong
Can you use this graph to help find Tf and use that to answer the equation
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- Magnesium sulfate is often used in first-aid hot packs, giving off heat when dissolved in water. A coffee-cup calorimeter at 25C contains 15.0 mL of water at 25C. A 2.00-g sample of MgSO4 is dissolved in the water and 1.51 kJ of heat are evolved. (You can make the following assumptions about the solution: volume=15.0 mL, density=1.00 g/mL, specific heat=4.18J/gC.) (a) Write a balanced equation for the solution process. (b) Is the process exothermic? (c) What is qH2O? (d) What is the final temperature of the solution? (e) What are the initial and final temperatures in F?If 5.0125 g of calcium chloride (CaCl2) is dissolved in 100.0 mL of water, the following data are collected: starting temperature is 22.5 °C, final temperature is 27.9 °C. Calculate the enthalpy of solution (∆Hsolution) in Joules. You may assume a density of 1.00 g/mL for the solution and specific heat is 4.18 J/(g×°C). Given: q = - m · Cp · ∆T and ∆Hsolution =q/moles soluteWhen 1.3 g of KClO3 was added to 151.1 g of water in a calorimeter, the temperature dropped by 0.588 °C. The heat capacity of H2O is 4.184 J/g°C. Assume the specific heat of the solution equals that of pure H2O and that the calorimeter neither absorbs nor leaks heat. What is the molar heat of the solution of solid potassium chlorate? Give the answer in kJ, but do not include the unit
- A student determines the heat of dissolution of solid ammonium chloride using a coffee-cup calorimeter of negligible heat capacity.When 2.84 g of NH4Cl(s) is dissolved in 109.00 g of water, the temperature of the solution drops from 25.00 to 23.20 °C. Based on the student's observation, calculate the enthalpy of dissolution of NH4Cl(s) in kJ/mol. Assume the specific heat of the solution is 4.184 J/g°C.ΔHdissolution = kJ/molIf 5.0125 g of calcium chloride (CaCl2) is dissolved in 100.0 mL of water, the following data are collected: starting temperature is 22.5 °C, final temperature is 27.9 °C. Calculate the enthalpy of solution (∆Hsolution) in Joules. You may assume a density of 1.00 g/mL for the solution and specific heat is 4.18 J/(g×°C). Given: q = - m · Cp · ∆T and ∆Hsolution =q/moles solute a.) is it exothermic or endothermic and how do you know?Sodium chloride is added in cooking to enhance the flavor of food. When 10.0 g of NaCl is dissolved in 200.0 mL of water at 25.0 0 C I a coffee‐cup calorimeter. 669 J of heat are absorbed. (You can make the following assumptions about the solution. Volume = 200.0 mL, density is 1.0 g/mL, specific heat capacity = 4.18 J/g 0 C. a) Is the solution process endothermic? b) What is q H2O ? c) What is the final temperature of the solution?
- 80.0 mL of isopropanol (d = 0.825 g/cm3) initially at 5.00°C was added to 60.0 mL of ethanol (d = 0.795 g/cm3) initially at 27.5°C. If the specific heat of ethanol is 2.46 J/g. oC and the specific heat of isopropanol is 2.68 J/g. oC), what was the final temperature of the solution once the liquids were mixed?A student determines the heat of dissolution of solid cobalt(II) chloride using a coffee-cup calorimeter of negligible heat capacity.When 1.52 g of CoCl2(s) is dissolved in 117.00 g of water, the temperature of the solution increases from 25.00 to 27.00 °C. Based on the student's observation, calculate the enthalpy of dissolution of CoCl2(s) in kJ/mol. Assume the specific heat of the solution is 4.184 J/g°C.ΔHdissolution = kJ/molMagnesium sulfate is often used in first-aid hot packs, giving off heat when dissolved in water. A coffee-cup calorimeter at 25°C contains 15.0 mL of water at 25°C. A 2.00-g sample of MgSO4 is dissolved in the water and 1.51 kJ of heat are evolved. (You can make the following assumptions about the solution: volume = 15.0 mL, density = 1.00 g/mL, specific heat 5 4.18 J/g · °C.)(a) Write a balanced equation for the solution process.(b) What is qH2O?(c) What is the final temperature of the solution?
- Determine the final temperature of a 32.3 mL salt solution initially at 21.05ºC when 1.93 kJ of heat is added to the solution. The specific heat of the solution is 3.264 J/(ºC · g) and the density of the solution is 1.235 g/mL. Report your answer in units of degrees Celsius to 4 sig figs, but do NOT include units in your answer.A calorimeter initially contains 165.0 mL of water at 21.3oC. When 2.72 g K is added to the water, the temperature of the resulting solution rises to a maximum of 47.8oC. The reaction that occurs is:2K(s)+H2O(l)→2KOH(aq)+H2(g) Assuming no heat exchange between the calorimeter and the surroundings, calculate the heat of reaction, qreaction, in joules. (Use the total solution mass for the calculation, assume the density of water is 1.00 g/mL and the specific heat of the solution is 4.18 J/(g oC). J What is the enthalpy change, ΔH, in kilojoules per mole of K? kJ/mol K What is the enthalpy change for the reaction, ΔHreaction? kJ/molSodium chloride is added in cooking to enhance the flavor of food. V\lnen10.00 g of NaCl are dissolved in 200.0 mL of water at 25.0°C in a coffee cup calorimeter, 669 J of heat are absorbed. (You can make the following assumptions about the solution: volume = 200.0 mL, density = 1.00" g/mL, specific heat = 4.18J/g . °C)(a) Is the solution process exothermic?(b) What is qH2O?(c) What isthe finaltemperature of the solution?

